Acute promyelocytic leukemia (APL) is a unique pathology among acute myeloid leukemias, with a high long-term survival compared to any other form of acute leukemia, but also with a high rate of early mortality due to coagulopathy associated with both hemorrhagic and thrombotic complications. We present the case of a patient with APL who developed acute coronary syndrome related to the complications of the hematological pathology, with a very good response at both hematological and cardiology treatments. This case emphasizes the importance of multidisciplinary approach in the case of a hematological patient.
Acute coronary syndrome – a rare complication in acute promyelocytic leukemia
Sindromul coronarian acut – o complicaţie rară în leucemia acută promielocitară
First published: 31 mai 2023
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/OnHe.63.2.2023.8093
Abstract
Rezumat
Leucemia acută promielocitară reprezintă o patologie unică printre leucemiile acute mieloide, cu o supravieţuire pe termen lung mai mare decât pentru orice altă formă de leucemie acută, dar şi cu o rată crescută a mortalităţii precoce, din cauza coagulopatiei asociate atât cu complicaţii hemoragice, cât şi cu complicaţii trombotice. Prezentăm cazul unui pacient diagnosticat cu leucemie acută promielocitară care a dezvoltat sindrom coronarian acut secundar complicaţiilor patologiei hematologice, cu răspuns favorabil atât la tratamentul hematologic, cât şi la cel cardiologic. Acest caz subliniază importanţa abordării multidisciplinare în situaţia unui pacient hematologic.
Introduction
Acute promyelocytic leukemia (APL) is a rare and aggressive subtype of acute myeloid leukemia, characterized by abnormal proliferation of promyelocytes, life-threatening coagulopathy and the specific chromosome translocation t(15;17)(q22;q11-12)(1). It represents a medical emergency with a high rate of early mortality(2), but as a result of the advances made in the treatment of this pathology, the long-term survival for patients with APL is higher than for any other form of acute leukemia(3). Taking this into account, we can even talk about curability(3,4).
Acute coronary syndrome and acute leukemias are rarely reported as concomitant conditions in the literature(5). Patients diagnosed with both have a worse prognosis than those with either condition alone(5), especially in those with acute promyelocytic leukemia which carries an additional risk for thrombosis. Due to complications of APL – namely, coagulopathy, thrombocytopenia and platelet dysfunction –, the management of acute coronary syndrome is challenging(6).
We present the case of a male patient with APL who developed acute coronary syndrome (ACS) during the treatment related to the complications of the hematological pathology. We review the possible mechanisms of ACS in the context of acute promyelocytic leukemia.
Case presentation
A 65-year-old Caucasian nonsmoker male patient, with cardiovascular risk factors, presented to the emergency department for rectorrhagia which had occurred two days before. The personal history included acute myocardial infarction (eleven years before presentation) treated with angioplasty, heart failure, arterial hypertension, type 2 diabetes and dyslipidemia. He was receiving medication for the chronic pathology.
The clinical examination was unremarkable, except for pallor and confirmed rectorrhagia, without other active hemorrhages – ECOG 3.
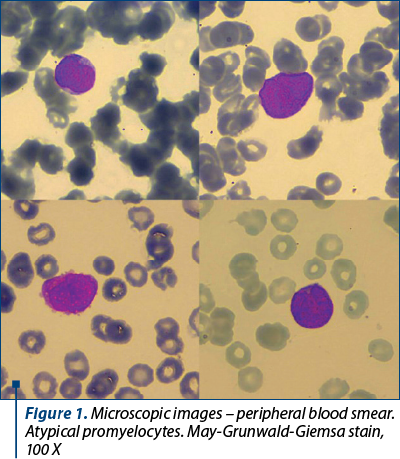
The laboratory tests revealed pancytopenia: moderate leucopenia – white blood cells (WBC) 2,900/mm3, severe normocytic anemia – hemoglobin (HGB) 7 g/dl, medium cell volume 95 fl, and severe thrombocytopenia – platelet count 7,000/mm3. Peripheral blood smear (Figure 1) identified atypical promyelocytes 30%, blasts 1%, neutrophils 2%, lymphocytes 26%, and monocytes 41%. Uric acid and creatinine were slightly increased. Also, aspartate aminotransferase was slightly increased, but viral markers were negative for hepatitis B and C, and for HIV infection.
The patient presented a decreased fibrinogen level (160 mg/dl), with slightly prolonged prothrombin time (international normalized ratio; PT INR) and normal activated partial thromboplastin time (aPTT).
The bone marrow aspiration revealed 66% atypical promyelocytes: large cells, with irregular nucleus, fine chromatin, some of them with nucleoli, basophilic cytoplasm with multiple granules. They expressed myeloperoxidase. Flowcytometry of bone marrow aspirate showed the presence of 39% precursor cells CD33+ cMPO+ CD117+ CD 34+ CD9+ CD123+ CD45RA+ CD2+/- CD10- CD3- CD7- cCD79a- CD19- CD56+/- CD11b- HLA-DR- and 31% precursor cells CD34+ CD117+ HLA-DR+ CD14- CD300e-/+ CD13- CD33++ CD11b+ CD36+ CD64+ CD4+ CD2+ CD9+. Thus, immunophenotyping showed two cell populations – one with 39% precursor cells of the myeloid lineage with a phenotype comparable to the atypical promyelocyte, and another with 31% precursor cells of the monocytoid lineage.
Fluorescence in situ hybridization identified the presence of t(15;17)(q24;q21) translocation. Molecular biology tests were positive for PML-RARa and for FLT3-ITD, and negative for NPM1.
Based on these findings, we established the positive diagnosis of APL with PML/RARa fusion, and FLT3-ITD positive (according to World Health Organization 2016 criteria), with intermediate risk.
The patient developed perirectal abscess complicated with fistula. We started intravenous broad-spectrum antibiotics, including for anti-anaerobe bacteria. He was also periodically evaluated by the surgeon. The evolution was favorable due to local (conservative) and systemic treatment.
Before the hematological treatment was started, an echocardiography was performed which revealed a left ventricular ejection fraction (LVEF) of 35% and kinetic impairment – akinesis of the apex, two apical thirds of the anterior wall and septum, and the apical third of the lateral wall.
According to the guidelines, we started specific treatment – namely, induction treatment with all-trans retinoic acid (ATRA), 45 mg/m2/day, in two divided doses, and arsenic trioxide (ATO) 0.3 mg/kg/d, days 1-5 in the first week, then two days a week, in weeks 2-8. Along with hematologic treatment, the patient received supportive treatment: transfusions with platelets and packed red blood cells, hemostatic agents, allopurinol, antibiotics and herpes zoster prophylaxis with acyclovir.
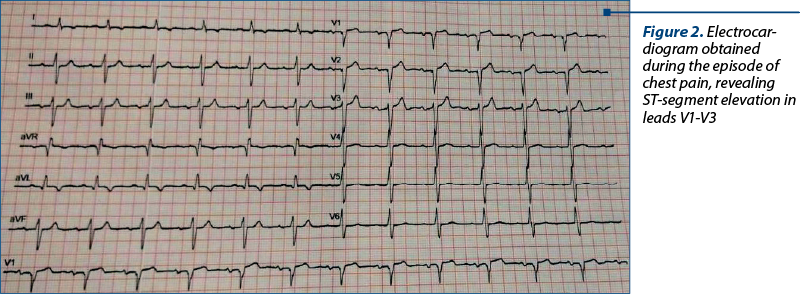
After the first two days of treatment with arsenic trioxide, the patient started to have severe anterior chest pain radiating in both arms and jaw, associated with difficulty in breathing and diaphoresis. An electrocardiogram revealed ST-segment elevation in leads V1-V3 (Figure 2). Laboratory analysis showed an increase of troponin-T from 29.6 ng/l initially to 400 ng/l (normal upper limit: 29 ng/l). The echocardiography showed decreased LVEF from 35% (at admission) to 25-30% and extended akinesia. A diagnosis of acute coronary syndrome and dilated cardiomyopathy was established.
The complete blood count showed leukocytosis (WBC 32,800/mm3), severe anemia (HGB 6.4 g/dl) and severe thrombocytopenia (platelet count 9,000/mm3). Coagulation panel revealed decreased fibrinogen (94 mg/dl) and increased prothrombin time of 22.3 seconds, INR 2.
The patient received oxygen, nitroglycerine and transfusion with packed red blood cells, taking into account that the severe anemia could aggravate the pain and the heart condition.
In this situation, the initial management includes the administration of antiplatelet agents and performing a coronary angiography. But, in our patient, neither of those could be accomplished because of the severe thrombocytopenia and coagulopathy. Thus, we completed his cardiology medication with angiotensin-converting enzyme inhibitor, beta-blocker, nitrates and statin. The patient was periodically evaluated by the cardiologist.
We decided to stop the arsenic trioxide administration and to continue the induction treatment with all-trans retinoic acid as a single agent, associated with steroids for the prevention of differentiation syndrome. Unfortunately, the diabetes decompensated and, besides the antidiabetic medication, the patient required insulin therapy during hospitalization.
We also continued the supportive treatment with intravenous broad-spectrum antibiotics, transfusions with platelets, packed red cells, fresh frozen plasma and cryoprecipitate, hemostatic agents and allopurinol.
The patient’s evolution was favorable. After the hematological features improved, we performed a bone marrow aspiration which revealed 3% normal promyelocytes, without any blasts, so the patient obtained complete hematological remission after induction therapy. He continued with consolidation treatment for intermediate-risk acute promyelocytic leukemia, with four courses of all-trans retinoic acid, 45 mg/m2/day for two weeks every 28 days, for seven cycles, and arsenic trioxide, 0.15 mg/kg/day, daily for five days per week, during weeks 1-4 of each eight-week cycle. Taking into account the recent history of acute coronary syndrome developed during induction, a complete evaluation was performed before every cycle of arsenic trioxide. Thus, besides hematologic specific tests, we evaluated the renal and hepatic function, the serum electrolytes, and made a complete cardiac evaluation – echocardiography before every cycle and electrocardiogram before and after every dose of arsenic trioxide to monitor QT interval. The patient was stable and did not develop any complication during the consolidation treatment. The remission was stable – molecular examination for PML-RARa was negative after induction and also after the consolidation treatment. He continued with maintenance treatment, preserving the complete molecular remission.
Discussion
A unique pathology among acute leukemias, acute promyelocytic leukemia differs from other subtypes of the class to which it belongs by its particular biology and cytogenetic features, targeted treatment and high curability. However, hemorrhagic complications and differentiation syndrome confer a high risk for early mortality in patients diagnosed with APL.
Approximately 95% of APL cases are characterized by t(15;17)(7), leading to PML-RARa fusion protein(8), which causes a dominant negative mutation(7), but it also has a gain-of-function effect(9,10). This blocks cell differentiation and prevents apoptosis, thus favoring leukemic cells proliferation(7).
Like any disease associated with medullary failure, most of the complications appear secondary to cytopenias(11), but some of them can be emphasized in the context of other processes. As an example, hemorrhagic complications are often out of proportion compared to the degree of thrombocytopenia(11).
The most feared and frequent complication of APL(12) is bleeding diathesis, representing the main cause of death, particularly at presentation(13,14), along with infections and differentiation syndrome(15). Early death remains the major cause of treatment failure in this type of highly curable acute leukemia(16,17). In APL, primary fibrinolysis is the main cause of hemorrhagic events, but it can also be triggered in the context of disseminated intravascular coagulation and secondary fibrinolysis(18). Rectal bleeding is rare among the initial signs of acute leukemia, but also represents a sign of severity(19).
Our patient presented with rectorrhagia, which was thought to be a consequence of hemorrhoids, thrombocytopenia and coagulopathy. While the rectal bleeding resolved, the patient quickly developed another complication, an infectious one – namely, perirectal abscess associated with fistula, those two representing the acute and chronic manifestation of the same disease, an infected anal gland(20). With careful surgical evaluation and proper treatment, the evolution was favorable.
In APL, due to unique mechanisms of coagulopathy, there is an increased risk of both hemorrhagic and thrombotic complications, the last one being underrecognized(12,21). Thrombotic events can occur either as a consequence of the expression of tissue factor and cancer procoagulant on the surface of leukemic promyelocytes, or as a component of the DIC spectrum(22,23). Thus, we can consider that activation of the fibrinolytic system is useful to some extent, as it breaks down the fibrin thrombus(18).
APL is the first hematological malignancy that benefits from molecular-targeted based therapy. Current guidelines recommend approaching the pathology as an emergency and ATRA initiation from the slightest suspicion of diagnosis(24,25). The standard of care for non-high-risk APL patients (WBC count ≤10,000/mm3) is ATRA plus ATO, this regimen being noninferior and possible superior to standard ATRA-idarubicin (AIDA) protocol(25).
In the case of our patient, considering the cardiac pathology, the history of myocardial infarction and the decreased LVEF, idarubicin could not be administered because of the cardiotoxicity of anthracyclines. Thus, according to guidelines, we decided for the combination between ATRA and arsenic trioxide, even though the last one also has potential cardiotoxicity. ATO treatment can cause QT interval prolongation, torsade de pointes and sudden cardiac death(26).
Major risk factors for acute coronary syndrome include male gender, smoking, hypertension, diabetes, dyslipidemia, obesity and family history of ACS(27-29).
Our patient’s risk for suffering an acute coronary syndrome was increased both by the hematological pathology and the underlying thrombotic risk, by severe anemia secondary to bone marrow failure, and worsened by rectal bleeding, as well as by cardiovascular risk factors, such as the personal history of acute myocardial infarction, hypertension, dyslipidemia, diabetes and obesity.
Several possible mechanisms of acute coronary syndrome in the context of APL have been described in literature.
During induction treatment for APL, the risk of thrombotic events is 5-20%(12). By impairing the balance between coagulation and fibrinolysis, it is possible that ATRA initially leads to a hypercoagulable status(30). Shortly after the initiation of ATRA, hyperfibrinolysis is corrected, but normalization of thrombotic markers usually occurs two weeks after the initiation of treatment(30). Thus, after starting the treatment, if fibrinolysis is disabled, there is an additional risk of thrombotic complications(18). Also, ATRA can cause coronary vasospasm(31,32).
The study of Chang from 2013 indicated one case of AMI out of ten patients with thrombotic events, who also associated gastrointestinal hemorrhage(21). Another study revealed that cardiac thrombotic events were among the most common thrombotic events before and during induction(23).
Also, directly related to the treatment with differentiation agents and as a possible mechanism of the ACS, we emphasize the differentiation syndrome(33), characterized by endothelial damage produced both by the release of IL-1b, IL-6, IL-8 and TNF-a by promyelocytes, following differentiation(34) and by cathepsin G, which increases vascular permeability(34). ATRA enhances the expression of certain adhesion molecules, which induces neoplastic cells aggregation(35). Endothelial lesions are precipitated by increased aggregation of blast cells and adhesion between blast cells and the endothelium(34). The clinical picture of differentiation syndrome implies dyspnea, pulmonary infiltrates, pleuro-pericardial effusion, peripheral edema, weight gain, fever and hypotension(36,37). The laboratory tests reveal leukocytosis and coagulopathy, and in severe cases, acute renal failure and hepatocytolysis(38). Our patient presented dyspnea, but we consider it in the context of acute pain and myocardial ischemia.
Disseminated intravascular coagulation leads to microthrombi in peripheral branches of the coronary arteries, which can cause severe cardiac dysfunction(31). This may be a possible mechanism of ACS in our patient, considering the increased INR and the low fibrinogen at the time of angina.
Another mechanism for acute coronary syndrome that leads to microthrombi formation through less activation of secondary fibrinolysis is elevated fibrinogen levels(39). It also plays a role in increasing clot stiffness, fiber density, platelet binding and blood viscosity(40).
Leukocytosis (white blood cells >10,000/mm3), CD2 expression of atypical promyelocytes, FLT3-ITD mutation and bcr3 isoform of the PML-RARa transcript are also associated with an increased risk for thrombosis in APL patients(41). Furthermore, related to leukocytosis, tissue infiltration by leukemic promyelocytes could be involved in the process(26). Our patient presented CD2 on the surface of leukemic promyelocytes and FLT3-ITD mutation.
A meta-analysis published in 2019 revealed that patients with FLT3-ITD have a higher risk of induction death and lower overall survival rates than those without mutation(42). Recent studies indicated that FLT3-ITD mutation did not confer a worse prognosis and did not influence the survival outcome in adult APL treated with ATO and ATRA-based therapeutic regimen(43-45).
Monotherapy with ATRA has been associated with frequent relapses, as continuous treatment with ATRA is characterized by a decrease in the plasma concentration of the drug secondary to accelerated clearance(46). Although our patient only received ATRA as induction therapy, he achieved complete remission and has maintained the response. An important aspect for maintaining the favorable response was applying consolidation treatment according to the guidelines.
Conclusions
Acute coronary syndrome is a rare complication associated with APL. In a patient presenting both conditions, choosing the best treatment option is challenging. Our case emphasizes the importance of close collaboration with other medical specialties in the management of a hematological patient with multiple comorbidities and complications secondary to the underlying pathology and treatment.

Conflict of interest: none declared
Financial support: none declared
This work is permanently accessible online free of charge and published under the CC-BY
Bibliografie
- Cingam SR, Koshy NV. Acute Promyelocytic Leukemia. 2022. Treasure Island (FL): StatPearls Publishing; 2023. PMID: 29083825.
- Larson RA, Gurbuxani S. Clinical manifestations, pathologic features, and diagnosis of acute promyelocytic leukemia in adults. UpToDate. 2022.
- Lo-Coco F, Avvisati G, Vignetti M, Thiede C, Orlando SM, Iacobelli S, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013; 369(2):111–121.
- Iland HJ, Bradstock K, Supple SG, Catalano A, Collins M, Hertzberg M, et al. All-trans-retinoic acid, idarubicin, and IV arsenic trioxide as initial therapy in acute promyelocytic leukemia (APML4). Blood. 2012;120(8):1570-1572.
- Ferrel MN, Ryan JJ, Han FT. Acute myeloid leukemia causing acute thrombosis of the coronary arteries: a case report. J Med Case Reports. 2022;16(1):149.
- Waterbury TM, Tarantini G, Vogel B, Mehran R, Gersh BJ, Gulati R. Non-atherosclerotic causes of acute coronary syndromes. Nat Rev Cardiol. 2020;17(4):229–41.
- Jimenez JJ, Chale RS, Abad AC, Schally AV. Acute promyelocytic leukemia (APL): a review of the literature. Oncotarget. 2020;11(11):992–1003.
- Poddighe PJ, Weghuis DO. t(15;17)(q24;q21) PML/RARA. Atlas Genet Cytogenet Oncol Haematol. 2016; 20(12):620-624.
- Lo-Coco F, Hasan SK. Understanding the molecular pathogenesis of acute promyelocytic leukemia. Best Pract Res Clin Haematol. 2014;27(1):3-9.
- Pandolfi PP. Oncogenes and tumor suppressors in the molecular pathogenesis of acute promyelocytic leukemia. Hum Mol Genet. 2001;10(7):769–775.
- Greer JP, Arber DA, Glader B, List AF, Means RT Jr, Paraskevas F, Rodgers GM. Wintrobe’s Clinical Hematology, 13th Ed., Lippincot Williams & Wilkins, 2013; pp. 3788-3822.
- Hambley BC, Tomuleasa C, Ghiaur G. Coagulopathy in Acute Promyelocytic Leukemia: Can We Go Beyond Supportive Care?. Front Med (Lausanne). 2021;8:722614.
- Yanada M, Matsushita T, Asou N, et al. Severe hemorrhagic complications during remission induction therapy for acute promyelocytic leukemia: incidence, risk factors, and influence on outcome. Eur J Haematol. 2007;78(3):213-219.
- de la Serna J, Montesinos P, Vellenga E, et al. Causes and prognostic factors of remission induction failure in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and idarubicin. Blood. 2008;111(7):3395-3402.
- Di Bona E, Avvisati G, Castaman G, et al. Early haemorrhagic morbidity and mortality during remission induction with or without all-trans retinoic acid in acute promyelocytic leukaemia. Br J Haematol. 2000;108(4):689-695.
- Choudhry A, DeLoughery TG. Bleeding and thrombosis in acute promyelocytic leukemia. Am J Hematol. 2012;87(6):596-603.
- Tallman MS, Lo-Coco F, Kwaan HC, Sanz MA, Gore SD. Early death in patients with acute promyelocytic leukemia. Proceedings from a live roundtable at the 2010 American Society of Hematology Annual Meeting, December 4-7, 2010, Orlando, Florida. Clin Adv Hematol Oncol. 2011;9(2 Suppl 3):1-16.
- Breen KA, Grimwade D, Hunt BJ. The pathogenesis and management of the coagulopathy of acute promyelocytic leukaemia. Br J Haematol. 2012;156(1):24-36.
- Fanta O, Ali I, Amadou DA, Youhanizou HM-L, Sahada MS, Nafissa A, Badé MA. Rectal Bleeding Revealing Acute Myeloid Leukemia (AML): About a Case in the Hepato-Gastroenterolgy Department of National Hospital of Niamey. Saudi J Med. 2022;7(12):607-609.
- Whiteford MH. Perianal abscess/fistula disease. Clin Colon Rectal Surg. 2007;20(2):102-109.
- Chang H, Kuo MC, Shih LY, et al. Acute promyelocytic leukemia-associated thrombosis. Acta Haematol. 2013;130(1):1-6.
- Sanz MA, Grimwade D, Tallman MS, et al. Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2009;113(9):1875-1891.
- Rashidi A, Silverberg ML, Conkling PR, Fisher SI. Thrombosis in acute promyelocytic leukemia. Thromb Res. 2013;131(4):281-289.
- Osman AEG, Anderson J, Churpek JE, et al. Treatment of Acute Promyelocytic Leukemia in Adults. J Oncol Pract. 2018;14(11):649-657.
- Sanz M, Fenaux P, Tallman M, Estey E, Löwenberg B, Naoe T, et al. Management of acute promyelocytic leukemia: updated recommendations from an expert panel of the European LeukemiaNet. Blood. 2019;133(15):1630-1643.
- Vineetha VP, Raghu KG. An Overview on Arsenic Trioxide-Induced Cardiotoxicity. Cardiovasc Toxicol. 2019;19(2):105-119.
- Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937-952.
- Murtaza Cheema F, Mujtaba Cheema H, Akram Z. Identification of risk factors of acute coronary syndrome in young patients between 18-40 years of age at a teaching hospital. Pak J Med Sci. 2020;36(4):821-824.
- Yadav P, Joseph D, Joshi P, Sakhi P, Jha RK, Gupta J. Clinical profile & risk factors in acute coronary syndrome. National Journal of Community Medicine. 2010;1(Issue-2):150-2.
- Ikezoe T. Pathogenesis of disseminated intravascular coagulation in patients with acute promyelocytic leukemia, and its treatment using recombinant human soluble thrombomodulin. Int J Hematol. 2014;100(1):27-37.
- De Santis GC, Madeira MI, de Oliveira LC, Falcao RP, Rego EM. Cardiac stunning as a manifestation of ATRA differentiation syndrome in acute promyelocytic leukemia. Med Oncol. 2012;29(1):248-250.
- Maqsood K, Mirrani G, Sarwar N, Burke JF. Coronary vasospasm mimicking ST-elevation myocardial infarction in a patient with ATRA-induced differentiation syndrome: a case report and review of literature. Journal of Hematology. 2013;2(1):27–30.
- Govind Babu K, Lokesh KN, Suresh Babu MC, Bhat GR. Acute Coronary Syndrome Manifesting as an Adverse Effect of All-trans-Retinoic Acid in Acute Promyelocytic Leukemia: A Case Report with Review of the Literature and a Spotlight on Management. Case Rep Oncol Med. 2016;2016:2829142.
- Stahl M, Tallman MS. Differentiation syndrome in acute promyelocytic leukaemia. Br J Haematol. 2019;187(2):157-162.
- Larson RS, Brown DC, Sklar LA. Retinoic acid induces aggregation of the acute promyelocytic leukemia cell line NB-4 by utilization of LFA-1 and ICAM-2. Blood. 1997;90(7):2747-2756.
- Sanz MA, Montesinos P. How we prevent and treat differentiation syndrome in patients with acute promyelocytic leukemia. Blood. 2014;123(18):2777-2782.
- Kota V, Kharkhanis P, Caprara CR, Bolds S, Debragga S, Simon KS, Arellano ML, Jillella A. Weight gain during induction therapy of acute promyelocytic leukemia patients: a preventable problem. Blood. 2017;130(1):5017.
- Montesinos P, Bergua JM, Vellenga E, et al. Differentiation syndrome in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and anthracycline chemotherapy: characteristics, outcome, and prognostic factors. Blood. 2009;113(4):775-783.
- Wada H, Mori Y, Okabayashi K, et al. High plasma fibrinogen level is associated with poor clinical outcome in DIC patients. Am J Hematol. 2003;72(1):1-7.
- Ariëns RA. Elevated fibrinogen causes thrombosis. Blood. 2011;117(18):4687-4688.
- Breccia M, Avvisati G, Latagliata R, et al. Occurrence of thrombotic events in acute promyelocytic leukemia correlates with consistent immunophenotypic and molecular features. Leukemia. 2007;21(1):79-83.
- Picharski GL, Andrade DP, Fabro ALMR, Lenzi L, Tonin FS, Ribeiro RC, Figueiredo BC. The impact of Flt3 gene mutations in acute promyelocytic leukemia: a meta-analysis. Cancers (Basel). 2019;11(9):1311
- Deka RR, Naseem S, Bhatia P, Binota J, Sonam P, Rana P, et al. FLT3-ITD mutation does not influence survival outcome in adult acute promyelocytic leukemia patients treated with ATO and ATRA-based therapeutic regimen: experience from a north indian tertiary care centre. Clin Lymphoma Myeloma Leuk. 2022;22(6):416-423.
- Iland HJ, Collins M, Bradstock K, et al. Use of arsenic trioxide in remission induction and consolidation therapy for acute promyelocytic leukaemia in the Australasian Leukaemia and Lymphoma Group (ALLG) APML4 study: a nonrandomised phase 2 trial. The Lancet Haematology. 2015;2(9):e357–66.
- Cicconi L, Divona M, Ciardi C, et al. PML-RARα kinetics and impact of FLT3-ITD mutations in newly diagnosed acute promyelocytic leukaemia treated with ATRA and ATO or ATRA and chemotherapy. Leukemia. 2016;30(10):1987–1992.
- Wang ZY, Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood. 2008;111:2505–2515.