Esenţialul despre imagistica prin rezonanţă magnetică multiparametrică în diagnosticul carcinomului hepatocelular
The essential of multiparametric magnetic resonance imaging in hepatocellular carcinoma diagnosis
Abstract
Hepatocellular carcinoma (HCC) is the most common primary malignant tumor of the liver, being strongly associated with cirrhosis, with a worldwide increasing incidence. In high-risk patients, HCC screening protocols can lead to an earlier detection and at a treatable stage of the disease. In this review, we will discuss and illustrate the Multiparametric Magnetic Resonance Imaging (MP MRI) evaluation in diagnosis of hepatocellular carcinoma, with emphasis on MRI protocol and HCC MRI features and aspects.Keywords
Multiparametric Magnetic Resonance Imagingdiagnosishepatocellular carcinomaRezumat
Carcinomul hepatocelular (CHC) este cea mai frecventă tumoră malignă primară a ficatului, asociată frecvent cu ciroza, cu o incidenţă crescândă la nivel mondial. Protocoalele de screening al CHC la pacienţii cu risc crescut pot duce la detectarea mai precoce şi într-un stadiu tratabil al bolii. În aceast review, vom discuta şi ilustra rolul Imagisticii prin Rezonanţă Magnetică Multiparametrică (IRM MP) în diagnosticul carcinomului hepatocelular, cu accent pe protocolul IRM şi pe caracteristicile şi aspectele CHC.Cuvinte Cheie
Imagistica prin Rezonanţă Magnetică Multiparametrică (IRM MP)diagnosticcarcinom hepatocelularIntroduction
Hepatocellular carcinoma (HCC) is the most common primitive hepatic tumor, occurring in 80-90% of cases in a context of liver cirrhosis (chronic hepatitis B and C virus infections), being four times more common in males, with a predilection in patients aged 50 to 70 years old. Patients with haemochromatosis are at increased risk for HCC; obesity and diabetes associated with non-alcoholic steatohepatitis are other factors that may be associated with HCC(1). Imaging, in particular Multiparametric Magnetic Resonance Imaging (MP MRI) represents a key element in the diagnostic algorithm and in the multidisciplinary customized management of each patient, allowing the number and size of tumoral nodules, their semiology, the involvement of intra- and extrahepatic vascular structures (portal venous structures – PV, hepatic veins – HV, inferior vena cava – IVC), the presence extrahepatic spread, the existence of anatomical variants or other incidentally discovered lesions(2-5). The prognosis over time is related to the size of the tumoral process, to the number of nodules, with HCC MRI semiology, the tumor extension, the possibility of PV or HV/IVC infiltration, and the “remaining” hepatic parenchymal function(1).
MP MRI evaluation technique
The MP MRI focused on the abdomen is performed with the patient in dorsal decubitus using a multi-channel phased array coil and machines with 1.5 or 3 T magnetic fields strength. In the MP MRI of the liver with specific hepatocyte contrast agent (Acidum Gadoxeticum-Gd-EOB-DTPA), after the three-plane locator, the dual T1 weighted image (wi) sequence with Echo Time (TE) in phase and opposition phase for detecting of lipomatous infiltrates and the hyper T2 wi sequence (with long TE) to exclude liver cysts and for the biliary tree global evaluation are performed, followed by the 3D T1 gradient echo (GRE) multi-phase acquisition pre- and postcontrast intravenous (i.v.) injection with hepatospecific paramagnetic gadolinium-based contrast agent (C.A.), injected dose of Acidum Gadoxeticum: 0.1 ml/kg body weight, after controlling the renal and the liver function tests results, using a low-dose rate injection, in late arterial phase (AP at approximately 30-35 seconds after the C.A. i.v. injection start) for the detection of hypervascularized liver nodules, portal venous phase (PVP at about 60-80 seconds after the C.A. i.v. injection start), and transient/late phase (TP at 120-150 sec after the C.A. i.v. injection start). In the waiting time frame, until the hepatobiliary phase (HBP) is performed – 20 minutes after the i.v. injection of a hepatospecific paramagnetic gadolinium-based C.A., we program and make the T2 ssFSE wi acquisitions with long and short TE that allow the differentiation between cysts and tissue/solid content of a lesion, Fast Spin Echo (FSE) T2 sequences with and without fat suppression (useful for detecting lipomatous focal liver lesions), the diffusion wi (the most sensitive sequence for hepatic lesions detection and for the assessment of high cellularity liver lesions in which free water diffusion is blocked due to the membranes cell abundance) and ADC map, the T2 GRE sequence – important in evaluating the iron content of the liver parenchyma and in highlighting the siderotic nodules, hemosiderin and hepatic calcifications(6-10). The hepatobiliary phase performed about 20 minutes after the i.v. injection of a hepatospecific contrast agent allows a superior characterization of liver lesions by tracing the border between benign and high-grade malignant tumors, detection of additional borderline lesions, as well as a global view on the hepatocyte function; in this phase, in patients with normal hepatocyte function, the liver parenchyma is in T1 FatSat wi homogeneous, discretely hyperintense, and the large intrahepatic bile ducts and main bile duct (MBD) are homogeneously opacified, with passage of the contrast into the second part of the duodenum (Figure 1). In order to assess the liver nodules enhancement, especially in cases of hyperintense T1 nodules detection in non enhanced MRI evaluation, subtraction is required, meaning native images are “subtracted” from post-contrast images acquired in the arterial phase (Figure 1).
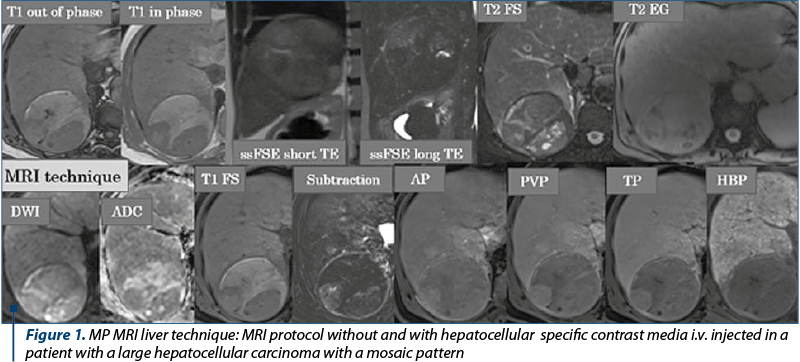
Semiological MRI aspects
The objectives of the hepatobiliary MP MRI are: the assessment of liver morphology and the degree of dysmorphia (left lobe hypertrophy, caudal lobe hypertrophy, right lobe and IV segment atrophy), detection and characterization of cirrhotic liver nodules and, respectively, highlighting HCC and its frame: single, multiple, infiltrative-diffuse, and its extent; the presence of portal hypertension signs; the appreciation of the anatomical variants at the level of the intra-/extrahepatic vascular axis and of the biliary tree; liver functional informations; follow-up of liver nodules with uncertain imaging pattern or of nodules treated by interventional radiology procedures(2-5,10-15).
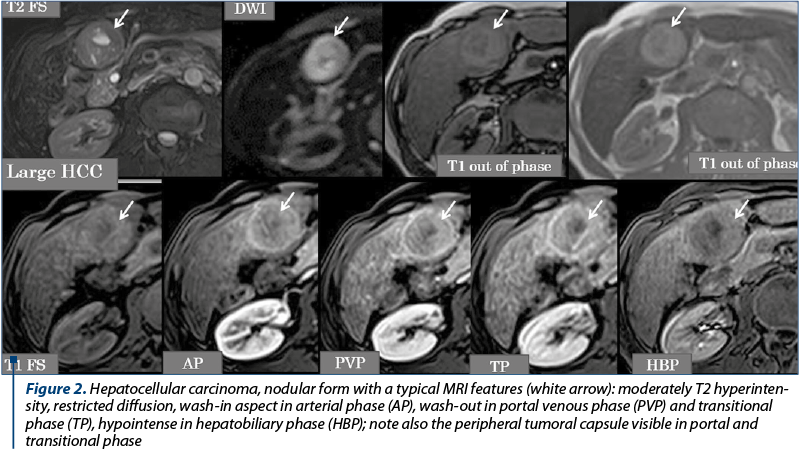
The imagistic aspects encountered in HCC are represented by: nodular form (unique nodule found in about 50% of cases), multinodular form – multiple tumor nodules (~40% of cases) and diffuse/infiltrative form. The current semiological criteria (LI-RADS: Liver Imaging Reporting and Data System for categories 4 and 5) that allow an accurate HCC diagnostic are: hyperenhancing liver mass in the arterial phase (wash-in), reflecting intra tumoral neoangiogenesis, which becomes hypoenhancing in portal venous phase and/or in transitional time (wash-out) by rapid venous drainage associated with reduced portal venous contribution in HCC (Figure 2), or having a dimensional increase equal to or greater than 50% of the initial dimensions in ≤6 months, or extends directly into the lumen of a venous liver structure: PV, HV, IVC (Figure 3). Other auxiliary criteria in favour of HCC are: identification of a non-enhancing capsule or pseudo-capsule in the periphery of nodules larger than 3 cm, visibility of the nodule on T2, T2 GRE and DWI wi, respectively hypointense round-oval lesion, on the ADC map(7,12,14,17), tumor nodules with dimensions of more than 3 cm, having a mosaic-like pattern structure given by the presence of necrotic, haemorrhagic components, rarely lipomatous or intrinsic calcifications, which alternate with solid areas(12). In the hepatobiliary phase, the vast majority of HCC nodules are T1 hypointense(7,8,14), due to the anaplasia and the hepatocyte dysfunction into the tumor (Figure 2). Tumoral thrombosis presents an identical semiology to the hepatic tumor (on unenhanced and enhanced MRI of the liver evaluation), characteristic being the wash-in in AP and the wash-out in the PV or TP(13). Particular forms of HCC are represented by hypoenhancing nodules in arterial phase, by the presence of T1 hyperintense nodules in HBP and the “nodule in nodule” appearance. For these situations, when the hepatic nodule is juxta-/infra-centimetric, the imaging follow-up over time is essential to see if the nodule progresses dimensionally. For lesions with dimensions equal to or greater than 2 cm, where there is a way of approach, a biopsy using ultrasound or CT guidance may be performed for histopathological framing(5,7,15,18).
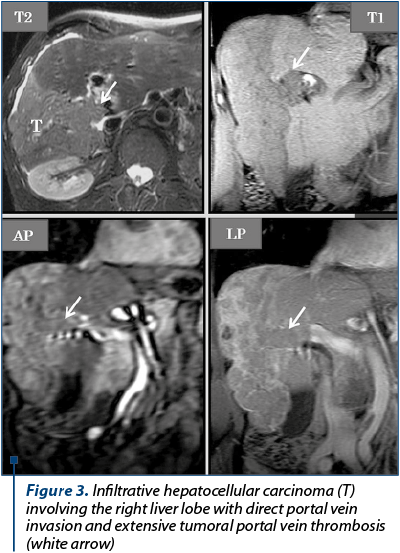
The differential diagnosis is performed with other hypervascular focal liver lesions(19): focal nodular hyperplasia (homogeneously enhancing mass on AP, isointense to the liver parenchyma in TP or in delayed phase, prolonged patchy hyperintense pattern of the entire FNH in HBP), adenoma (miming HCC in the context of hormonal treatment, bulky forms often have haemorrhagic components), hemangioma (cyst-like T2 hypersignal lesion with centripetal, progressive and persistent contrast enhancement in PVP and LP), hypervascularized metastases (multiple, usually small sized hypervascular lesions in AP, with wash-out in PVP, in a patient with a history of neoplasia), cholangiocarcinoma (capsular retraction, BD dilatation, peripheral contrast enhancement with central hypointense zone in AP, peripheral hypointense rim and central enhancing area in LP/HBP corresponding to fibrotic component – targetoid aspect), arterio-portal shunts (they are precociously visible in AP and become isointense to the liver parenchyma in PVP, TP and HBP) – Table 1.

Conclusions
The MP MRI with hepatospecific paramagnetic gadolinium-based contrast agent, centered on the abdomen, is the imaging modality of choice to evaluate liver cirrhotic nodules. The role of the radiologist in the clinical suspicion of HCC grafted on a cirrhotic liver is to make a positive diagnosis and to participate, according to the MRI semiological aspects, in the individualized multidisciplinary therapeutic decision approach for each patient, and in cases of an “atypical” nodule pattern, to propose a different imaging lesion follow-up or a targeted intralesional hepatic biopsy. The imaging report should contain a complete description of the hepatic nodule (s), of its complications (portal thrombosis; metastasis-lymph nodes, pulmonary, bone dissemination), anatomical variants, and other extrahepatic lesions discovered incidentally.
Conflict of interests: The author declares no conflict of interests.
Bibliografie
- Balogh J, Victor D, Asham EH, et al. Hepatocellular carcinoma: a review. Journal of Hepatocellular Carcinoma. 2016; 3: 41–53.
- Willatt JM, Hussain HK, Adusumilli S, Marrero JA. MR imaging of hepatocellular Carcinoma in the cirrhotic liver: challenges and controversies. Radiology. 2008; 247:311–330.
- Hanna RF, Diego AA, Kased N, et al. Cirrhosis-associated hepatocellular nodules: correlation of histopathologic and MR imaging features. RadioGraphics. 2008; 28:747–769.
- Choi JY, Lee J-M, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part I. Development, growth, and spread: key pathologic and imaging aspects. Radiology. 2014; 272:635–654.
- Choi JY, Lee JM, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part II. Extracellular agents, hepatobiliary agents, and ancillary imaging features. Radiology. 2014; 273:30–50.
- Neri E, Bali MA, Ba-Ssalamah A et al .ESGAR consensus statement on liver MR imaging and clinical use of liver-specific contrast agents. Eur Radiol. 2016; 26:921–931.
- LI-RADS- CT/MRI LI-RADS® v2018 CORE, www.acr.org
- Lee SE, An C, Hwang SH, et al. Extracellular contrast agent-enhanced MRI: 15-min delayed phase may improve the diagnostic performance for hepatocellular carcinoma in patients with chronic liver disease. Eur Radiol. 2018; 28:1551–1559.
- Di Martino M, Di Miscio R, De Filippis G, et al. Detection of small (≤2 cm) HCC in cirrhotic patients: added value of diffusion MR-imaging. Abdom Imaging. 2013; 38:1254–1262.
- Lupescu IG. Hepatic nodules in cirrhosis.A-366, Insight into Imaging. ECR 2012 book of abstracts, S431; EPOS™.DOI 10.1594/ecr/2012/A-366.
- Kartik Jhaveri K, Cleary S, Audet P, et al. Consensus Statements From Multidisciplinary Expert Panel on the Utilization and Application of a Liver-Specific MRI Contrast Agent (Gadoxetic Acid). AJR. 2015; 204:498–509.
- Cannella R, Furlan A. Mosaic architecture of hepatocellular carcinoma. Abdom Radiol. 2018; 43:1847–1848.
- Matteo Renzulli M, Brocchi S, et al. Can Current Preoperative Imaging Be Used to Detect Microvascular Invasion of Hepatocellular Carcinoma. Radiology. 2016; 279 (2): 432-442.
- Joo I, Lee JM, Lee DH, et al. Noninvasive diagnosis of hepatocellular carcinoma on gadoxetic acid-enhanced MRI: can hypointensity on the hepatobiliary phase be used as an alternative to washout? Eur Radiol. 2015; 25:2859–2868.
- Arslanoglu A, Seyal AR, Sodagari F, et al. Current Guidelines for the Diagnosis and Management of Hepatocellular Carcinoma: A Comparative Review. AJR. 2016; 207:W88–W98.
- Choi MH, Choi J, Lee YL, et al. MRI of Small Hepatocellular Carcinoma: Typical Features Are Less Frequent Below a Size Cutoff of 1.5 cm. AJR. 2017; 208:544–551.
- McNamara MM, Thomas JV, Alexander LF. Diffusion-weighted MRI as a screening tool for hepatocellular carcinoma in cirrhotic livers: correlation with explant data - a pilot study. Abdom Radiol. 2018; https://doi.org/10.1007/s00261-018-1535-y
- Sanghvi T Boyum J, Spilseth B, et al. MRI for hepatocellular carcinoma: a primer for magnetic resonance imaging interpretation. Abdom Radiol. 2018; 43:1143–115; https://doi.org/10.1007/s00261-017-1280-7
- Kim TK, Lee E, Jang HJ. Imaging findings of mimickers of hepatocellular carcinoma. Clin Mol Hepatol. 2015; 21:326–343.



