Hepatitis B virus – a silent pathogen with many faces of chronic infection. Case report
Virusul hepatitei B – un agent patogen silenţios cu mai multe feţe ale infecţiei cronice. Prezentare de caz
Abstract
Chronic hepatitis B is a major cause of morbidity and mortality worldwide, especially with vertical (mother-to-child) and horizontal transmission in early childhood. Although it is difficult to diagnose in children, partly because most of them are in the immunotolerant-phase but also due to the nonspecific symptoms and to a lack of evidence from low-income countries with a high frequency of hepatitis B virus (HBV) infection to guide pediatric care, it is a major problem of public health, causing chronic liver disease and even hepatocarcinoma. We report the cases of five brothers with their mother infected with HBV, all being placed in foster care. The children’s age was between 5 and 13 years old and they were asymptomatic. The laboratory findings revealed hepatitis B surface antigen (HBsAg) positive, increased viral load (>2000 copies/ml) and slightly increased transaminases in all patients but with hepatitis B e antigen (HBeAg), anti-hepatitis B core antibodies (anti-HBc) and anti-hepatitis B e antibodies (anti-HBe) different in each and one of them. After diagnosis, the initiation of treatment was considered, but none of the patients met the criteria for the antiviral treatment available in our country. The patients were managed conservatively, with regular monitoring of transaminases and serology, and had a good evolution.Keywords
hepatitis B virushepatitischronicchildrenRezumat
Hepatita cronică cu virus B este o cauză majoră de mortalitate şi morbiditate la nivel mondial, mai ales atunci când modalitatea de transmitere este verticală (de la mamă la făt) sau orizontală, în special în copilărie. Chiar dacă este rar diagnosticată în populaţia pediatrică, pe de-o parte datorită faptului că majoritatea pacienţilor sunt în faza de imunotoleranţă a bolii, iar pe de altă parte, din cauza simptomatologiei nespecifice şi a absenţei datelor colectate din statele endemice pentru hepatita B, ţări cu programe deficitare de informare populaţională cu privire la răspândirea şi diagnosticarea infecţiei, reprezintă o problemă majoră de sănătate publică, în timp conducând la afectare cronică hepatică şi cu posibile complicaţii precum ciroza şi hepatocarcinomul. Prezentăm cazurile a cinci fraţi, toţi proveniţi din mamă purtătoare de AgHBs cu replicare virală, instituţionalizaţi atât în serviciul de stat, cât şi în plasament la asistenţi sociali. Vârsta pacienţilor este cuprinsă între 5 şi 13 ani, iar simptomele de la momentul prezentării au fost nespecifice. Investigaţiile paraclinice au decelat antigenul de suprafaţă al hepatitei B (AgHBs) pozitiv la toţi pacienţii, viremie crescută (>2000 copii/mL) şi niveluri uşor crescute ale transaminazelor, dar cu AgHBe, anticorpi anti-HBc şi anticorpi anti-HBe diferiţi de la un caz la altul. Imediat după momentul diagnosticării, s-a luat în considerare iniţierea tratamentului, dar niciunul dintre pacienţi nu a îndeplinit criteriile necesare pentru tratamentul antiviral disponibil în ţara noastră. În acest caz, pacienţii au fost manageriaţi conservator, cu monitorizări regulate ale transaminazelor şi ale markerilor serologici, cu o evoluţie excelentă de-a lungul multiplelor monitorizări efectuate în serviciul nostru.Cuvinte Cheie
virus hepatic BhepatităcroniccopiiIntroduction
Chronic hepatitis B is one of the leading causes of cirrhosis and liver transplantation in the modern era in adults. It is a debilitating disease that may last for decades if acquired at a young age. Although a partial seroconversion can be revealed in some cases, most patients experience active viral replication. They are at a higher risk of developing cirrhosis, with a later onset of the hepatocarcinoma(1-3). The predominant transmission route in the highly endemic countries is vertical (from mother to child), whereas in the low-endemic countries the most common way of transmission is parenteral. Hepatitis B virus (HBV) chronic infection is defined by the persistence of hepatitis B surface antigen (HBsAg) for at least six months. It is divided into the immunotolerant phase (high viral replication accompanied by hepatitis B e antigen [HBeAg] production, with normal transaminases and no i mmune-mediated damage – most common in children), the immune clearance phase (HBeAg positive, fluctuating transaminases, high serum HBV-DNA), the inactive carrier phase (HBeAg positive, low serum HBV-DNA and normal transaminases), the immune reactive phase (anti-HBe antibodies positive, moderate or fluctuating serum HBV-DNA levels, elevated or fluctuating transaminase levels), and the HBsAg-negative phase (loss of HBsAg, undetectable HBV-DNA in the serum, but detectable in the liver, normal transaminases)(4).
In terms of treatment, two main agents are frequently used: interferon (IFN) and nucleos(t)ide analogs (NA). Even though none of the anti-HBV drugs currently available can be considered curative or eradicative for HBV, they are the only option to control the course of the disease(5). In addition, there are several criteria for initiating the treatment: HBsAg carrier for at least six months, more than 2 years of age, HBeAg positive, elevated transaminases (more than 1.5 times the upper limit of normal), positive HBV-DNA (more than 2000 copies/ml) and abnormal histology (with the mention that the liver biopsy is not mandatory). Vaccination is the most efficient path to eradicate chronic hepatitis B infection(6).
Case report
We report the cases of five brothers, all of them HBsAg-positive, none of them with positive medical history, but with a family history of chronic HBV infection. The children were between 5 and 13 years old, and the sex ratio was 3:2 (male:female). The symptoms were nonspecific, starting with fatigue, abdominal pain or even no clinical manifestations, with no signs of peritoneal irritation, arthralgia, jaundice, itchy skin, hepatomegaly, portal hypertension or ascites. On admission, the laboratory findings revealed that they were HBsAg-positive and negative for anti-HBs antibodies.
Since the definition of chronic hepatitis B requires a six-month persistence of the HBsAg, they have been followed-up. At the second appointment, all of them were diagnosed with chronic hepatitis B, most of them with similar laboratory findings (Table 1).
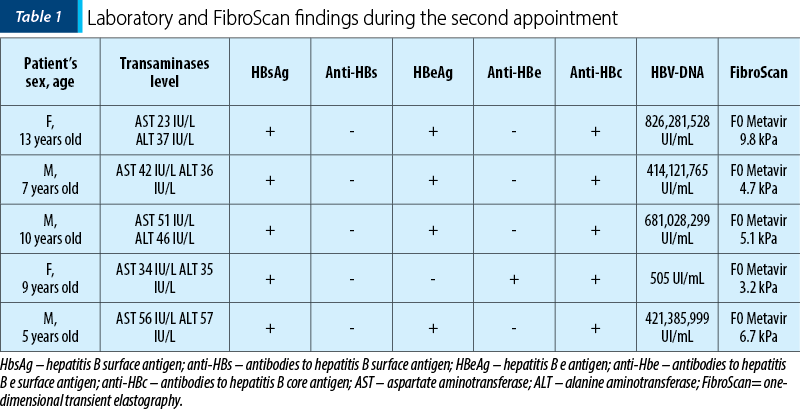
Only one of the patients had normal levels of transaminases (alanine aminotransferase [AST] 34 IU/L, aspartate aminotransferase [ALT] 35 IU/L), with negative HBeAg and positive anti-HBe. The rest were all positive for HBeAg and negative for anti-HBe, with slightly to moderately elevated transaminase levels (AST between 36 to 51 IU/L and ALT between 35 to 68 IU/L). Furthermore, analyzing the viral load, we noticed that in the first case there was a decreased level of HBV-DNA copies/ml (505 UI/mL) compared to the other children who had HBV-DNA levels between 414,121,765 UI/mL and 826,281,528 UI/mL. We performed one-dimensional transient elastography (FibroScan) for each patient, which revealed no sign of fibrosis, with a hepatic rigidity between 3.2 kPa and 9.8 kPa, equivalent to a hepatic rigidity of the F0 Metavir stage. In addition, laboratory findings revealed normal immunoglobulin G (IgG) levels in all patients with negative antinuclear antibodies (ANA) and negative Epstein-Barr virus (EBV) antibodies, cytomegalovirus (CMV) antibodies or hepatitis C (HCV) antibodies.
Since the children were diagnosed with chronic hepatitis B, the next step was the attempt to start the treatment as soon as possible, not only to shorten the course of the disease but also to control the activity, the necrosis progression and inflammation to prevent the evolution of fibrosis, cirrhosis and eventually hepatocellular carcinoma in the future adulthood. Taking into consideration the inclusion criteria for initiating the treatment, we analyzed each case, especially concerning the patient’s age, the transaminase level (over 1.5 x normal values for at least six months), the viral load and the assessment of fibrosis and necroinflammatory activity (using the Fibrotest). Unfortunately, none of our patients met the criteria for initiating the treatment, partly because the transaminase levels were only slightly increased, with no sign of necroinflammatory activity. Even though, for the moment, the option to initiate the treatment with antiviral agents is not suitable for our patients, we still monitor them regularly, with the measurement of transaminase levels, antigens and antibodies and also the viral load at intervals of six months or sooner, in case of recurrence of alarm symptoms such as jaundice, ascites, hepatomegaly or increased fatigue.
Discussion
According to the new terminology adopted by the European Association for the Study of the Liver (EASL) in 2017, chronic HBV infection can be characterized based on the presence or absence of active hepatitis (defined as increased or normal aminotransferase levels, respectively) and on the HBeAg status(7). The decision to initiate the treatment should be evaluated at each follow-up to use antiviral drugs at the earliest signs of liver damage. The first patient, a 9-year-old female, HBsAg-positive, with normal transaminase levels and low viral load, also had a negative HBeAg and positive anti-HBe antibodies. She was in the inactive carrier phase (or immune-control phase), with no necroinflammatory activity and no evidence of any progression to cirrhosis. The treatment was not indicated, so the only therapeutic option was to monitor the viral load, the transaminase levels and the liver function tests(8). These patients have inactive disease, but in some cases they reactivate into an HBeAg-negative immune-active phase, while others revert to the immune-active phase (HBeAg-positive)(9). Concerning prognosis, it is excellent as long as the viral load remains low, the transaminase levels are normal and the anti-HBe antibodies are positive. The next patient was a 13-year-old female, HBsAg-positive, with slightly elevated transaminase levels and very high HBV-DNA level. In addition, the HBeAg was positive and she did not have anti-HBe antibodies. She was in the immunotolerant phase in which the liver disease is none to minimal, and there was no progression to cirrhosis. Even though the viral load was very high, the transaminase levels were only slightly increased. Therefore, postponing the treatment was the best option for her.
Furthermore, the literature shows that patients with lower to normal transaminases have fewer chances of achieving serological response(10). As well as in the first case, our remaining option was to follow her up periodically. If the viral load remains just as high and the transaminase levels increase, we will initiate the treatment with antiviral drugs.
The remaining cases were similar to the last one, with HBsAg-positive, HBeAg-positive, negative anti-HBe antibodies, slightly to moderately elevated transaminase levels and very high HBV-DNA levels (from 414,121,765 UI/mL to 681,028,299 UI/mL). Like before, they were in the immunotolerant phase of the disease, including patients with criteria for initiating the treatment but with no inflammatory activity and no signs of progression to cirrhosis. Even though the viral load was very high and the age criterium was met, we did not initiate the treatment because there was no evidence of active liver disease. The efficacy of the antiviral drugs would be low. Furthermore, the treatment of these patients is not recommended because the patients are unlikely to respond, and the treatment with nucleoside or nucleotide analogs may induce drug resistance and even cross-resistance to newer medications(11). So, again, our therapeutic option was to monitor them periodically; in the presence of high transaminase levels (especially ALT), the next step is the assessment of viral load, as high HBV-DNA values warrant antiviral treatment, whereas low levels should support investigations to exclude other causes of liver disease(12,13). Once again, we realize the importance of regular monitoring of our patients, which ultimately leads to a more favorable course of the disease and to the prevention of further complications associated with chronic hepatitis B.
In terms of treatment, as listed above, our therapeutic options are the interferon (IFN) and nucleos(t)ide analogues (NA) – entecavir and tenofovir. Even though the IFN treatment is a serious option considering the time-defined cycle of therapy (6 to 12 months) and the absence of risk of viral resistance, the injectable format and also the serious adverse effects such as flu-like symptoms, nausea, myalgia, diarrhea, alopecia, medular suppression and autoimmune phenomena (vasculitis, Raynaud’s phenomenon, systemic lupus erythematosus, rheumatoid arthritis) make it less desirable in comparison with the NA(14). On the other hand, the NAs have the advantage of oral administration and few side effects. Still, the unknown duration of therapy and the high resistance rate compared to the interferon makes them inconvenient for certain patients(15). In the EASL review of the leading studies of entecavir and tenofovir, anti-HBe seroconversion occurred in 21% of patients, compared with 30% for IFN. Subsequent HBsAg loss occurred only in 2% of patients and in 3%, respectively. Furthermore, a reduction in viral load to less than 60-80 IU/mL was seen in 67% and 76% of those treated, significantly higher than that achieved with IFN(16,17). Yet again, the decision to initiate the treatment and the optimal management of hepatitis B in children are complex and challenging.
A particularity of these children is that they acquired the infection vertically from their natural mother, who presented chronic hepatitis B infection. The first step in preventing vertical transmission of HBV is to test all pregnant women in the first trimester to identify the best management strategy for mothers and the correct immunoprophylaxis schedule for future newborns(18). After exposure, the risk of chronicity is higher for newborns (90%), infants and children younger than 5 years old (25-30%) than for adolescents or adults (<5%)(19). The pregnancies were not correctly monitored in their cases, without periodic checks or laboratory tests performed throughout pregnancy. Consequently, no prophylaxis was administered at birth, which resulted in them being infected with the hepatitis B virus.
Additionally, after a short period spent at home, the children were placed in foster care. They were considered healthy, without any symptoms present, with no additional investigations regarding their family history, and were diagnosed at a distance from their birth. Once again, we highlight the importance of prevention, education and screening campaigns in disadvantaged, low-income environments, as most of the patients from these regions have a higher risk of developing long-term complications.
Even though chronic hepatitis B is a treatable disease, especially when detected in the early stages, few patients are at risk of rapid disease progression and early development of complications, with a quarter of infected individuals developing severe complications in adult life(20). Our purpose as pediatricians is to prevent, diagnose and analyze the treatment options and the prognosis for each patient to shorten the course of the disease and prevent further complications associated with chronicity. Having a clear image of the replication phases and criteria for initiating the treatment, as well as the periodicity of monitoring are the key to success, since chronic hepatitis B can be misleading in some of the intermediate phases.
Conclusions
Chronic hepatitis B is one of the most challenging medical situations in pediatrics, especially when the patients come from disadvantaged environments, with poor knowledge of the disease itself and its complications. An important aspect highlighted by these cases is that early diagnosis, active screening during pregnancy and educational campaigns in low-income regions should be a common practice among clinicians in order to reduce hepatitis B transmission and prevent long-term morbidity. Furthermore, regular monitoring and individualized treatment should be applied for a better quality of life.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
-
Indolfi G, Easterbrook P, Dusheiko G, et al. Hepatitis B virus infection in children and adolescents. Lancet Gastroenterol Hepatol. 2019;4(6):466-476. doi: 10.1016/S2468-1253(19)30042-1. Erratum in: Lancet Gastroenterol Hepatol. 2020;5(5):e4
-
Sokal E. Drug treatment of pediatric chronic hepatitis B. Paediatr Drugs. 2002;4(6):361-9. doi: 10.2165/00128072-200204060-00003
-
Defresne F, Sokal E. Chronic hepatitis B in children: Therapeutic challenges and perspectives. J Gastroenterol Hepatol. 2017;32(2):368-371. doi: 10.1111/jgh.13459
-
Davison S. Management of chronic hepatitis B infection. Arch Dis Child. 2014;99(11):1037-42. doi: 10.1136/archdischild-2013-304925.
-
Dusheiko G. Treatment of HBeAg positive chronic hepatitis B: interferon or nucleoside analogues. Liver Int. 2013;33 (S1):137–50
-
European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–98.
-
European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–98.
-
Sokal EM, Conjeevaram HS, Roberts EA, et al. Interferon alfa therapy for chronic hepatitis B in children: a multinational randomized controlled trial. Gastroenterology. 1998;114:988–995.
-
Terrault NA, Bzowej NH, Chang KM, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016; 63:261.
-
Jonas MM, Kelly DA, Mizerski J, et al. Clinical trial of lamivudine in children with chronic hepatitis B. N Engl J Med. 2002;346:1706–1713.
-
American Academy of Pediatrics. Management and Prevention of Infectious Diseases. In: Red Book: 2021-2024 Report of the Committee on Infectious Diseases, 32nd Ed, Kimberlin DW, Barnett ED, Lynfield R, Sawyer MH (Eds), American Academy of Pediatrics, Itasca, IL 2021. p.122.
-
Hsu EK, Murray KF. Hepatitis B and C in children. Nat Clin Pract Gastroenterol Hepatol. 2008;5:311–320.
-
Shah U, Kelly D, Chang M-H, et al. Management of chronic hepatitis B in children. J Pediatr Gastroenterol Nutr. 2009;48:399–404.
-
Piratvisuth T, Marcellin P, Popescu M, et al. Hepatitis B surface antigen: association with sustained response to peginterferon alfa-2a in hepatitis B e antigen-positive patients. Hepatol Int. 2013; 7 (2):429-36.
-
Iorio R, Pensati P, Botta S, et al Side effects of alpha-interferon therapy and impact on health-related quality of life in children with chronic viral hepatitis. Pediatr Infect Dis J. 1997;16(10):984-90.
-
Chang TT, Gish RG, de Man R, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354:1001–10.
-
Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359:2442–55.
-
European Association for the Study of the Liver.; European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398.
-
Day FL, Karnon J, Rischin D. Cost-effectiveness of universal hepatitis B virus screening in patients beginning chemotherapy for solid tumors. J Clin Oncol. 2011;29:3270–7.
-
Sokal EM, Paganelli M, Wirth S, et al. European Society of Pediatric Gastroenterology, Hepatology and Nutrition. Management of chronic hepatitis B in childhood: ESPGHAN clinical practice guidelines: consensus of an expert panel on behalf of the European Society of Pediatric Gastroenterology, Hepatology and Nutrition. J Hepatol. 2013;59(4):814-29. doi: 10.1016/j.jhep.2013.05.016.




