According to a study realized at Johns Hopkins University, up to 50% of the patients have nutritional deficiences when they are hospitalized, and only 20% of the patients benefit from a nutritional consult at the admission. This situation is seen in patients of all ages, but older people are very vulnerable. The nutritional deficit significantly increases the risk of postoperative complications and, consequently, the risk of death after surgery, being the main reason for hospital readmission. This is due to the lack of sufficient biological resources to deal with the catabolism determined by the systemic inflammatory response. Cancer, other chronic diseases and the surgical intervention trigger a systemic inflammatory reaction. This inflammatory response, whose intensity is determined by the extent of the surgical act, leads to the intensification of glycogen and lipid catabolism, with the release of glucose, free fatty acids and amino acids in the circulation. The routine nutritional screening in all oncology patients planned for surgery, followed by preoperative nutritional therapy, is essential for preventing postoperative complications.
Importanţa screeningului nutriţional la pacientul chirurgical oncologic
Importance of nutritional screening in the surgical oncology patient
First published: 27 martie 2019
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/OnHe.46.1.2019.2308
Abstract
Rezumat
Potrivit unui studiu realizat la Universitatea Johns Hopkins, din SUA, până la 50% dintre pacienţi au deficienţe nutriţionale la internarea în spital şi numai aproximativ 20% dintre pacienţi sunt supuşi unui consult nutriţional la admiterea în spital. Această situaţie se întâlneşte la toate vârstele, însă persoanele în vârstă sunt deosebit de vulnerabile. Deficitul nutriţional creşte semnificativ riscul de complicaţii postoperatorii şi, implicit, riscul de deces după intervenţia chirurgicală, fiind principalul motiv pentru care pacienţii sunt reinternaţi în spital. Acest lucru se datorează lipsei de resurse biologice suficiente pentru a face faţă catabolismului declanşat de răspunsul inflamator sistemic. Cancerul, alte boli cronice şi intervenţia chirurgicală declanşează o reacţie inflamatorie sistemică. Această reacţie inflamatorie, a cărei intensitate este influenţată de amploarea actului chirurgical, duce la intensificarea catabolismului glicogenului, a lipidelor şi, în lipsa rezervelor energetice, şi a proteinelor, cu eliberarea în circulaţie de glucoză, acizi graşi liberi şi de aminoacizi. Efectuarea screeningului nutriţional de rutină la toţi pacienţii cu cancer care urmează a fi supuşi unei intervenţii chirurgicale, urmată de terapia nutriţională preoperatorie, este esenţială pentru prevenirea complicaţiilor postoperatorii.
Introduction
Surgery in patients with digestive neoplastic diseases is performed in a complex pathological context determined by: the oncological disease in different evolution stages; multiple comorbidities; precarious nutritional status induced, on one hand, by the consumptive neoplasia and the metabolism deflected towards a hypercatabolic reaction, and, on the other hand, by the limitted alimentary intake due to inapetence, vomiting and digestive losses.
Among different localizations of digestive cancers, the gastric and esophageal neoplasm is associated with more severe alterations of the nutritional status.
The surgical stress determines significant endocrine, metabolic and immunological reactions. Proteic and lipid hypercatabolism, the diminishing of insulin’s anabolic effect and insulin resistance, on one hand, and the inflammatory reaction with the increase of IL-6, IL-1, TNF and C-reactive protein(1), on the other hand, can extremely affect the oncology patient. The effects of this process are the increase in energy consumption, increase of proteolysis, protein structural modifications, hyperglycemia, and extravasation of liquid from cells to the extracellular space(2). Both surgery and anesthesia can alter the defense mechanisms and can depress the natural killer cells activity, essential for the destruction on neoplastic cells(3).
Many local and general factors have been incriminated in the apparition of anastomotic fistulas. The local factors are related to the surgical act, in this category being included the advanced stage of the tumor(4), the extended resections and the emergency surgery(5). The general factors are represented by: male sex(4-6), age, increased ASA risk(5), diabetes mellitus(6), other associated pathologies, and nutritional status at the admission.
Nutritional status. The nutritional screening
The nutritional status has been recognised as being important in influencing the postoperative morbidity since 1936, when Studley published data on the relationship between weight loss and mortality after gastroduodenal surgical interventions. In recent years, this has been taken into consideration as an independent risk factor that can influence the apparition of digestive fistulas(7,8).
Therefore, it follows that the nutritional state at admission is a factor which significantly influences the postoperative outcome for every type of surgery, especially in patients with increased risk and other risk factors. In these patients, a proper surgical technique in the absence of a preoperative preparation to correct the biological constants and the nutritional status cannot reduce the incidence of fistulas(9-11). The surgical act triggers an inflammatory reaction whose intensity is dependent on the extent of the surgical intervention. The inflammatory process triggers a metabolic response consisting in the increase on the energy consumption. The systemic inflammatory response generated by surgery is mediated by cytokines and has a major impact on metabolism, with the intensification of glycogen and lipid catabolism and, in the absence of energy reserves and proteins, with the release of glucose, free fatty acids and amino acids in the circulation. Apart from metabolic and endocrine reactions, the surgical stress determines important immunological reactions.
ERAS (Enhanced Recovery After Surgery) recommendations(12), initially created for improving recovery after colorectal oncological surgery, have been extended to other types of interventions. The protocol includes among others:
the limitation of preoperative abstinence for clear liquids at two hours, and at six hours for food, respectively;
the administration of carbohydrates per os (sweet liquids) in the evening before surgery and two hours before the intervention;
the avoidance of liquid overload both intraoperatively and postoperatively;
the precocious mobilization;
the rapid introduction of the enteral alimentation (in the first 24 hours);
the avoidance of opioids for pain relief, due to their effects of prolonged postoperative ileus.
These measures applied in the perioperative period are not enough for preventing the postoperative complications in patients with preoperative weight loss.
In gastric surgery, ERAS protocol(13) reccomends to introduce oral or enteral nutritional support beginning with the preoperative period.
In order to establish the degree of nutritional deficit, ESPEN guides support the assessment of nutritional status even from the patient’s admission and the weekly reevaluation(14), using NRS 2002 (Nutritional Risc Score).
At the patient’s admission, there must be carried out the initial screening which consists in questioning the patient regarding involuntary weight loss in the last three months, whether he had appetite loss in the last week or he suffers from severe medical conditions with ICU (Intensive Care Unit) admission, and the BMI (Body Mass Index) is calculated – Table 1.
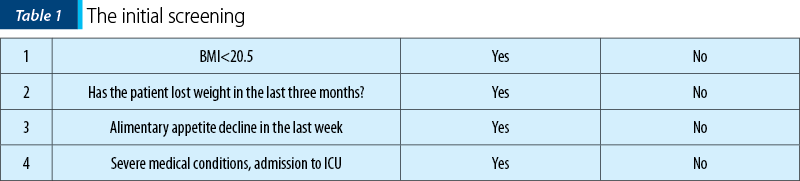
If the answer is ‘No’ to all these questions, the screening will be repeated after one week. If the answer is ‘Yes’ to all these questions, the patient is considered with increased nutritional risk.
In hospitalized patients there is carried out the final screening, which has two components: nutritional status and disease severity.
For the nutritional status, the scoring system is as follows: score 0 if the patient didn’t lose weight and has normal appetite; score 1 if the patient has lost more than 5% of the body weight in the last three months and the alimentary intake has been below 50-70% of the necessities in the last week; score 2 if the patient has lost more than 5% of the body weight in the last two months or has a BMI between 18.5 and 20 associated with the altering of the general condition and an alimentary intake between 25% and 60% of the necessities; score 3 if the patient has lost more than 5% of the body weight in one month or more than 15% in three months or he has a BMI less than 18.5 associated with the altering of the general condition and an alimentary intake below 25% of the normal level (Table 2).
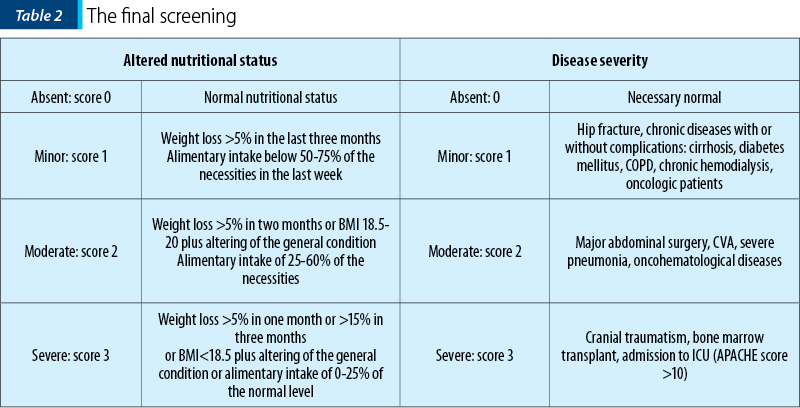
For assessing the disease severity, the scoring system is as follows: score 0 for a minor disease; score 1 for patients with hip fracture, chronic diseases with or without complications (cirrhosis, diabetes mellitus, COPD, chronic hemodialysis, oncology patients); score 2 in major abdominal surgery, cerebral vascular accident (CVA), severe pneumonia, oncohematological diseases; score 3 in patients with cranial traumatism, bone marrow transplant, admission to ICU (APACHE score >10) – Table 2.
For patients older than 70 years, one point is added to the final score.
Interpretation: a score ≥3 means a patient with nutritional risk who necessitates a therapy plan; a score <3 means a patient who necessitates weekly reevaluation.
This draws the attention to the relationship between preoperative malnutrition and the rate of complications and postoperative mortality. The nutritional deficit is often undiagnosed(14).
NRS is validated as predictive factor of morbidity associated to surgical interventions by prospective studies (EuroOOPS, 2008; Schwegler, 2010)(14). Weight loss and decreased albumin level (<3 g/dl) are other parameters with a good predictive value(10). Another study (Kuppinger, 2012), cited by ESPEN in 2017(14), revealed a good appreciation of the nutritional status using as assessing parameter only the appetite decline before hospitalization in patients who underwent abdominal surgery. Other authors use modified nutritional screening scores(12).
The concept of preparation before surgery enters in another era, becoming an essential stage for preventing postoperative complications. The assessment of nutritional status and its optimization with nutritional therapy introduced 10-14 days before surgery in patients with insufficient alimentary intake don’t associate significant biological modifications. Some authors recommend a program of “prehabilitation”, which includes physical exercises and respiratory kinetotherapy in order to improve the muscular function(14).
It is important to underline that in patients without denutrition it is not indicated the protein calorie supplementation even after surgery, if the restart of a sufficient per os administration is expected in less than seven days.
In other patients, an energetic supplementation of 25 kcal/kg and 1.5 g of amino acids in 24 hours, preferably on enteral administration, must be continued until the alimentation will provide more than 60% of the necessities.
Finally, we must take into consideration that not all oncology patients with malnutrition can receive nutritional therapy. In esogastric cancers especially, but also in cancers with other localizations, different complications of the disease can be associated, such as digestive hemorrhages, stenoses, occlusions, massive digestive fluid losses, and tumor necroses with associated sepsis. In these cases, only minimal re-equilibrations are allowed, the temporization of surgery leading to the supplementary alteration of the patient’s condition rather to improving the metabolic status. The pathological context represented by comorbidities, such as heart failure, hepatic insufficiency, uncontrolled diabetes mellitus, blood dyscrasias and shock, can limit or contraindicate the protein calorie support, either parenteral or enteral.
Conclusions
Performing the routine nutritional screning in all oncology patients planned for surgery, followed by preoperative nutritional therapy, is essential for preventing postoperative complications.
Conflict of interests:
Bibliografie
- Pullicino EA, Carli F, Poole S, Rafferty B, Malik STA, Elia M. The relationship between the circulating concentrations of interleukin 6 (IL-6), tumor necrosis factor (TNF) and the acute phase response to elective surgery and accidental injury. Lymphokine Research. 1991; vol. 9, no. 2, 231–238.
- Mueller C, Compher C, Druyan ME; the American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors. A.S.P.E.N. Clinical Guidelines Nutrition Screening, Assessment, and Intervention in Adults. Journal of Parenteral and Enteral Nutrition. 2011 January; Volume 35 Number 1, 16-24.
- Snyder GL, Greenberg S. Effect of anaesthetic technique and other perioperative factors on cancer recurrence. Br. J. Anaesth. 2010; 105:106–115.
- Park JS, Choi GS, Kim SH, Kim HR, Kim NK, Lee KY, Kang SB, Kim JY, Lee KY, Kim BC, Bae BN, Son GM, Lee SI, Kang H. Multicenter analysis of risk factors for anastomotic leakage after laparoscopic rectal cancer excision: the Korean laparoscopic colorectal surgery study group. Ann. Surg. 2013 Apr; 257(4):665-71.
- Bakker IS, Grossmann I, Henneman D, Havenga K, Wiggers T. Risk factors for anastomotic leakage and leak-related mortality after colonic cancer surgery in a nationwide audit. Br. J. Surg. 2014 Mar; 101(4):424-32, discussion 432.
- Zhang W, Lou Z, Liu Q, Meng R, Gong H, Hao L, Liu P, Sun G, Ma J, Zhang W. Multicenter analysis of risk factors for anastomotic leakage after middle and low rectal cancer resection without diverting stoma: a retrospective study of 319 consecutive patients. Int. J. Colorectal Dis. 2017 Oct; 32(10):1431-1437.
- Kwag SJ, Kim JG, Kang WK, Lee LK, Oh ST. The nutritional risk is a independent factor for postoperative morbidity in surgery for colorectal cancer. Ann Surg Treat Res. 2014 Apr; 86(4): 206–211.
- Lee SY, Jung MR, Kim CH, Kim YJ, Kim HR. Nutritional risk screening score is an independent predictive factor of anastomotic leakage after rectal cancer surgery. European Journal of Clinical Nutrition. 2018 Apr; 72(4):489-495.
- Marimuthu K, Varadhan KK, Ljungqvist O, Lobo DN. A meta-analysis of the effect of combinations of immune modulating nutrients on outcome in patients undergoing major open gastrointestinal surgery. Ann Surg. 2012 Jun; 255(6):1060-8.
- Yeh DD, Fuentes E, QUrashi SA, Cropano C, Kaafarani H, Lee J, et al. Adequate nutrition may get you home: effect of caloric/protein deficits on the discharge destination of critically ill surgical patients. J. Parenter. Enteral. Nutr. 2016; 40:37e44(5).
- Gillis C, Carli F. Promoting perioperative metabolic and nutritional care. Anesthesiology. 2015; 123:1455e72.
- Feldheiser A, Aziz O, Baldini G, Cox BP, Fearon KC, Feldman LS, Gan TJ, Kennedy RH, Ljungqvist O, Lobo DN, Miller T, Radtke FF, Ruiz Garces T, Schricker T, Scott MJ, Thacker JK, Ytrebø LM, Carli F. Enhanced Recovery after Surgery (ERAS) for gastrointestinal surgery, part 2: consensus statement for anaesthesia practice. Acta Anaesthesiol Scand. 2016 Mar; 60(3):289-334.
- Mortensen K, Nilsson M, Slim K, Schäfer M, Mariette C, Braga M, Carl F, Demartines N, Griffin SM, Lassen K. Consensus guidelines for enhanced recovery after gastrectomy: Enhanced Recovery After Surgery (ERAS) Society recommendations. Br J Surg. 2014 Sep; 101(10):1209-29.
- Weimann A, Braga M, Carli F, Higashiguchi T, Hübner M, Klek S, Lavian A, Ljungqvis O. Lobo DN, Martindale R, Waitzberg DL, Bischoff SC, Singer P. ESPEN guideline: Clinical nutrition in surgery. Clinical Nutrition. 2017; 36, 623-650.
Articole din ediţiile anterioare
Metastaze skip şi supravieţuirea - argumente pentru disecţia ganglionilor limfatici în cancerul pulmonar
Lung cancer continues to be the most common cause of death related to cancer per year and the number is increasing every year. One case out of 10 i...
Iron, ferroptosis and association with tumoral evolution and potential therapeutic impact
Articolul îşi propune să arate tendinţele moderne de redefinire a rolului fierului în procesele metabolice normale şi patologice, reamintind ...
Faslodex in second line of hormonal treatment of advanced breast cancer - case report
Acest caz se referă la o pacientă cu cancer mamar avansat local şi cu metastaze osoase multiple. Pacienta a suferit o intervenţie chirurgicală pali...
Rolul nutriţiei în cancer
Factorii alimentari joacă un rol complex în etiopatogeneza cancerelor umane. Se apreciază că dieta, inactivitatea fizică şi obezitatea sunt respons...