At the moment, genetic tests have become an important and necessary part of the diagnosis and treatment algorithm of cancer. Genetic testing becomes mandatory in many cancers for the correct orientation of treatment. Genetic tests have a prognostic and predictive role, depending on the outcome of the treatment and the prognosis group in which the patient is framed. Therefore, we can speak at this moment about custom therapies tailored to each individual patient, depending on the molecular biology of the tumor, prognostic factors and predictive factors. Targeted therapies are selected according to the mutations identified at the tumor level, which offer a major advantage in terms of response rate, time to disease progression and median survival. Also, the benefit of adjuvant therapies or certain types of cytotoxic agents may be estimated by preliminary genetic testing, thereby avoiding additional toxicity in patients with good prognosis at low risk of recurrence. Also, genetic tests can be used to determinate the risk of developing cancer. Several types of cancer display a familial predisposition and specific gene mutations confer a high-lifetime risk to develop the disease. During the last decades, the basis for such genetic predisposition has been clarified for several cancer syndromes and the high-risk genes mutated in familial cases are currently subjected to genetic diagnostic screening programs. Mutation testing in these genes has a major impact in genetic counseling, helps increase the chance of survival, defines the prognosis of carriers, and identifies the most appropriate and prophylactic measures.
Importanţa testării genetice în cancer
The importance of genetic testing in cancer
First published: 20 decembrie 2017
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/OnHe.41.4.2017.1347
Abstract
Rezumat
În momentul de față, testele genetice au devenit o parte importantă și necesară din algoritmul de diagnostic și tratament al cancerului. Testarea genetică devine astfel obligatorie în multe tipuri de cancer, pentru orientarea corectă a tratamentului. Testele genetice au rol prognostic și predictiv, rezultatul ajutând la stabilirea tratamentul în funcție de grupa de prognostic în care este încadrat pacientul. Prin urmare, putem vorbi în momentul de față de terapii personalizate, adaptate fiecărui pacient în parte, în funcție de biologia moleculară a tumorii, de factorii de prognostic și de factorii predictivi. Terapiile țintite sunt selecționate în funcție de mutațiile identificate la nivel tumoral, oferind un avantaj major în ceea ce privește rata de răspuns, timpul până la progresia bolii și supraviețuirea mediană. De asemenea, beneficiul terapiilor adjuvante sau al anumitor tipuri de agenți citotoxici poate fi estimat prin teste genetice preliminare, evitând astfel toxicități suplimentare la pacienții cu prognostic favorabil, cu risc scăzut de recurenţă. Totodată, testele genetice pot fi utilizate pentru a identifica riscul unui individ de a dezvolta cancer. Pacienții cu predispoziție ereditară de a dezvolta un anumit tip de cancer sunt ulterior orientați către programele de screening corespunzătoare, consiliere genetică și măsuri profilactice individualizate.
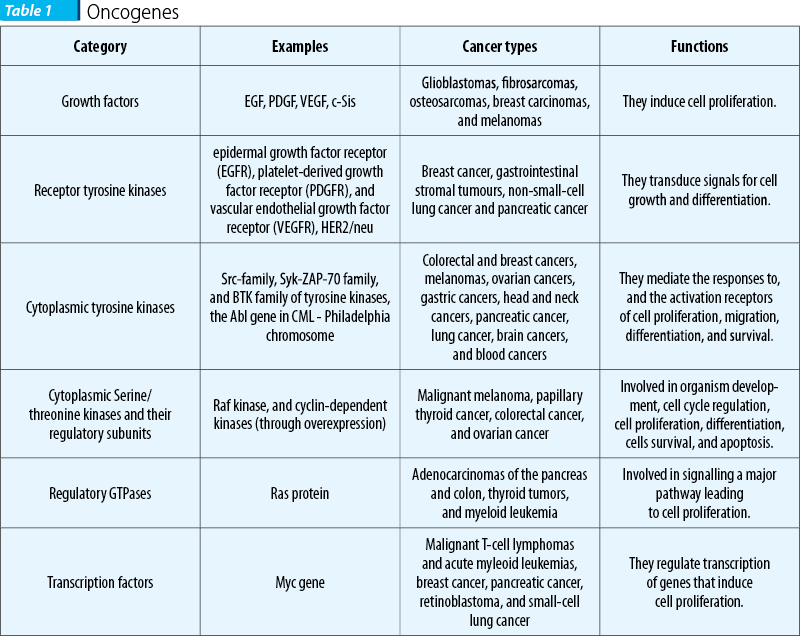
Cancer arises from the accumulation of genetic abnormalities in somatic cells, through altered gene expression like mutations, chromosome defects, and epigenetic aberrations. Over 500 genes are now known to be involved in cancer development. The basic mechanism in cancer is mutation, which means changing in DNA sequence. These mutations can be inherited or acquired during lifetime through the influence of carcinogenetic factors(1). Mutations can be: germline mutations (familial cancer), that can appear in 5-10% of all cancer cases, being present in every cell of the body, and passed from parent to child; or sporadic (somatic) mutations, caused by carcinogenetic agents exposure, which are not in every cell and are not passed from parent to child. There are three types of carcinogenesis due to carcinogenic factors: carcinogenesis due to physical agents - ionizing radiation; chemical carcinogenesis - exposure to chemical agents; and biological carcinogenesis - exposure to biological agents like viruses and some parasites(2). Somatic mutations in cancer genome are a consequence of multiple processes including deficiencies in DNA repair, endogenous and exogenous carcinogen exposure, and enzymatic alterations in DNA. The normal regulatory genes are the target of genetic damage: the growth promoting proto-oncogenes, the growth inhibiting tumor suppressor genes, genes that regulate programmed cell death (apoptosis), and DNA repair genes(3).
Proto-oncogenes are normal genes involved in the growth and proliferation of cells, and also in the process of apoptosis. Proto-oncogenes encode proteins, growth factors or their receptors, signal transducers, transcription factors, and cell cycle components.
Oncogenes are mutated and activated proto-oncogenes. The activation of oncogenes is achieved through mutations, translocations, retroviral insertions or amplifications. Theodor Boveri was the first to describe oncogenes in 1914, in his work titled Theory of oncogenes. Later, he published another work, titled The Origin of Malignant Tumors, in which is suggested the link between oncogenes and malignant tumors. First oncogene (sarcoma oncogene) was described in 1970 by G. Steve Martin from the University of California, Berkeley. Later, in 1976, J.M. Bishop and H.E. Varmes suggested that oncogenes were activated proto-oncogenes, an observation for which they won the Nobel Prize(4).
Proto-oncogenes activation occurs in three ways: translocation or transposition, point mutations and gene amplification. These genetic changes lead to a triggering of a cascade of cellular events with major impact in cell proliferation and survival.
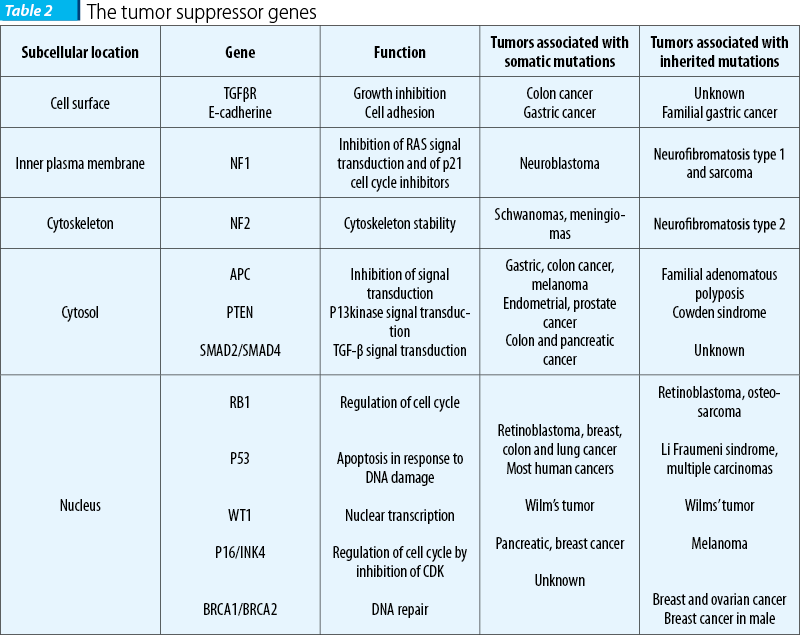
Tumor suppressor genes are protective genes. Normally, they limit cell growth by monitoring how quickly cells divide into new cells, repairing mismatched DNA, and controlling apoptosis. When the function of both alleles is lost, malignant transformation can occur (most mutations are recessive)(5).
The tumor suppressor gene p53 is called “the guardian of the genome” and is located on 17p13 chromosome. More than 50% of human cancers carry mutations of p53 gene. If damaged DNA cannot be repaired, gene p53 initiates the apoptotic process. If the gene carries a mutation, the abnormal cell can escape the apoptosis. The presence of the p53 mutations can be a predictive factor for chemotherapy and radiotherapy. Retinoblastoma tumor suppressor gene, located on 13q14 chromosome, inactivates E2F (a transcription factor) and inhibit cell cycle in phase G1. Tumor antigen (derived from viruses) may combine with RB protein and can lead to uncontrollable and continuous cell division(6).
DNA repair genes fix mistakes made when DNA is copied. If a person has an error in a DNA repair gene, these mistakes are not corrected and mutations can occur, which may eventually lead to cancer. Mutations in DNA repair genes can be inherited (Lynch syndrome) or acquired(7).
Uses of genetics in cancer risk, diagnosis and treatment:
- Risk of cancer
- Early diagnosis
- Classification of cancer
- Metastasis
- Biology of malignant tumor
- Evolution of the cancer
- Monitoring treatment
- Progression and response to therapy
- Drug target
- Drug resistance
- DNA repair processes
- Mechanism of DNA damage(8).
Genetic testing for cancer risk
Genetic testing looks for specific inherited mutations in a person’s chromosomes, genes or proteins. Inherited mutations are thought to play a role in about 5 to 10 percent of all cancers. Cancer can sometimes appear to “run in family”. A shared environment or lifestyle, such as tobacco use, can cause similar cancers to develop among family members. However, certain patterns - such as the types of cancer that develop, other non-cancer conditions that are seen, and the ages at which cancer typically develops - may suggest the presence of a hereditary cancer syndrome. The genetic mutations that cause many of the known hereditary cancer syndromes have been identified. Finding a characteristic mutation can confirm whether a condition is the result of an inherited syndrome. Genetic testing is also done to determine in the same family if a member without obvious illness has inherited the same mutation as a family member who is known to carry a cancer-associated mutation. Inherited genetic mutations can increase a person’s risk of developing cancer, but is not a mandatory condition(9).
Several types of cancer display a familial predisposition, and specific gene mutations confer a high-lifetime risk to develop the disease. During the last decades, the basis for such genetic predisposition has been clarified for several cancer syndromes and the high-penetrant/ high-risk genes mutated in familial cases are currently subjected to genetic diagnostic screening programmes. Mutation testing in these genes has a major impact in genetic counseling, helps increase the chance of survival, defines the prognosis of carriers and identifies the most appropriate and personalized prophylactic measures(10).
More than 50 hereditary cancer syndromes have been described.
-
Hereditary breast cancer and ovarian cancer syndrome
- Genes: BRCA1, BRCA2
- Related cancer types: female breast, ovarian and other cancers, including prostate, pancreatic, and male breast cancer.
-
Li-Fraumeni syndrome
- Gene: TP53
- Related cancer types: breast cancer, soft tissue sarcoma, osteosarcoma (bone cancer), leukemia, brain tumors, adrenocortical carcinoma (cancer of the adrenal glands), and other cancers.
-
Cowden syndrome (PTEN hamartoma tumor syndrome)
- Gene: PTEN
- Related cancer types: breast, thyroid, endometrial (uterine lining), and other cancers
-
Lynch syndrome (hereditary nonpolyposis colorectal cancer)
- Genes: MSH2, MLH1, MSH6, PMS2, EPCAM
- Related cancer types: colorectal, endometrial, ovarian, renal, pancreatic, liver and biliary tract, stomach, brain, and breast cancers(9).
-
Familial adenomatous polyposis
- Gene: APC
- Related cancer types: colorectal cancer, multiple non-malignant colon polyps, and both non-cancerous (benign) and cancerous tumors in the intestine, brain, stomach, bone, skin.
-
Retinoblastoma
- Gene: RB1
- Related cancer types: eye cancer (cancer of the retina), pinealoma (cancer of the pineal gland), osteosarcoma, melanoma, and soft tissue sarcoma.
-
Multiple endocrine neoplasia type 1 (Wermer syndrome)
- Gene: MEN1
- Related cancer types: pancreatic endocrine tumors, parathyroid and pituitary gland tumors.
-
Multiple endocrine neoplasia type 2
- Gene: RET
- Related cancer types: medullar thyroid cancer and pheochromocytoma (benign adrenal gland tumor).
-
Von Hippel-Lindau syndrome
- Gene: VHL
- Related cancer types: kidney cancer and multiple noncancerous tumors, including pheochromocytoma.
Hereditary breast and ovarian cancer (HBOC) and Lynch syndrome are among the most widely studied cancer syndromes. The high penetrant BRCA1 and BRCA2 susceptibility genes for breast and ovarian cancer were discovered between 1994 and 1995. Subsequent genetic studies based on linkage and positional cloning helped identify additional moderate-risk genes, and genome-wide association studies identified common low penetrance alleles associated with breast cancer heritability. In Lynch syndrome, four mismatch repair (MMR) genes confer high-penetrance for colorectal and endometrial cancer onset (MLH1, MSH2, MSH6, PMS2), and intensive research explored and identified additional genetic risk variants(9).
How is genetic testing done?
Genetic tests are usually requested by a person’s doctor or other health care provider. Testing is done on a small sample of body fluid or tissue - usually, blood, but sometimes saliva, cells from inside the cheek, skin cells or amniotic fluid (the fluid surrounding a developing fetus). The sample is then sent to a laboratory specialized in genetic testing. The laboratory returns the test results to the doctor or genetic counselor who requested the test. It usually takes several weeks or longer to get the test results. Genetic counseling is recommended both before and after genetic testing to make sure that patients have accurate information about what a particular genetic test means for their health and care. A “positive test result” means that the laboratory found a specific genetic alteration (or mutation) that is associated with a hereditary cancer syndrome. A positive result may:
- confirm the diagnosis of a hereditary cancer syndrome;
- indicate an increased risk of developing certain cancer(s) in the future;
- show that someone carries a particular genetic change that does not increase their own risk of cancer, but that may increase the risk of their children (if the child also inherits an altered copy from the other parent);
- suggest a need for further testing;
- provide important information that can help other family members make decisions about their own health care(10).
A “negative test result” means that the laboratory did not find the specific alteration that the test was designed to detect. A negative result can show that the tested family member has not inherited the mutation that is present in their family. The patient does not have an increased genetic risk of developing cancer, nor is he a carrier of a mutation that increases cancer risk. A negative result does not mean that there is no cancer risk, but rather that the risk is probably the same as the cancer risk in the general population.
Genetic testing has limitations and emotional implications: depression, anxiety, or guilt, personal and family tension. A positive test result means a gene mutation is detected and some people with positive tests may think of themselves as sick, even if they never develop cancer. A patient with a negative result can have a false sense of security. A negative result means that a person doesn’t have a specific genetic mutation. However, a person with a negative result may still develop cancer. A negative result only means the person’s risk is average. Additionally, each person’s risk is affected by lifestyle, environmental factors, and medical history.
Who should have genetic testing? Genetic counseling and testing may be recommended for people who have had certain cancers or certain patterns of cancer in their family:
- Several first-degree relatives with cancer.
- Many relatives on one side of the family who have had the same type of cancer.
- A family member with more than one type of cancer.
- Family members who had cancer at a younger age than normal for that type of cancer.
- A family member with a rare cancer, such as breast cancer in a male or retinoblastoma.
- A physical finding that’s linked to an inherited cancer (such as having many colon polyps).
- A known genetic mutation in one or more family members who have already had genetic testing(11).
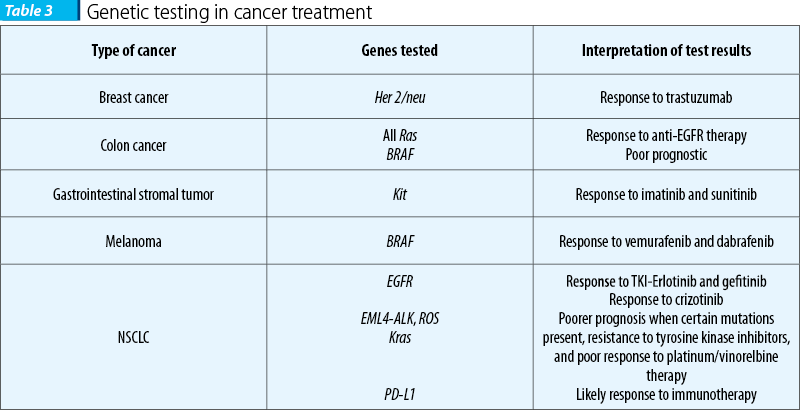
Genetic testing in cancer treatment
Genetic tests in cancer treatment are important for the following aspects:
- Cancer drugs that inhibit or target very specific proteins associated with certain cancers (examples are tyrosine kinase inhibitors and epidermal growth factor receptor [EGFR] antibodies).
- Genetic tests to detect the presence of mutations in cancer tissue that tell a health care practitioner whether the person being tested is likely to benefit from a specific therapy.
- Progression and response to therapy.
- Drug target.
- Drug resistance.
Genetic testing in breast cancer
Her2 amplification in breast cancer
The tyrosine kinase receptor erbB-2 is a protein that in humans is encoded by the ERBB2 gene. It is also frequently called HER2 (from human epidermal growth factor receptor 2) or HER2/neu. HER2 is a member of the human epidermal growth factor receptor (HER/EGFR/ERBB) family. Amplification or over-expression of this oncogene has been shown to play an important role in the development and progression of certain aggressive types of breast cancer. Her2/neu amplification represents a prognostic and predictive factor and can be determined by immunohistochemistry or fluorescence in situ hybridization (FISH). The meaning of gene amplification is the response to anti-Her2 therapy: trastuzumab (Herceptin®) monoclonal antibody, pertuzumab (Perjeta) monoclonal antibody, ado-trastuzumab emtansine (Kadcyla®, also known as TDM-1), a monoclonal antibody attached to a chemotherapy drug, and lapatinib (Tykerb®), a tyrosinkinase inhibitor(11).
Oncotype test in breast cancer
The Breast Recurrence Score test is a genomic test looking at the activity of tumor genes. Specifically, the test measures the activity of a group of cancer-related genes in tumor tissue. The activity of these genes can provide information about how tumor might behave in the future, including how likely is to grow and spread or whether it is likely to respond to chemotherapy. This is a prognostic and predictive test which analyzes the activity of 21 genes, the results reflecting the risk of metastases development/ recurrence of the disease, and estimating the benefit of adjuvant chemotherapy. The results of oncotype test combined with other features of the tumor can give information about whether to have chemotherapy to treat early stages, hormone-receptor positive, Her2 negative breast cancer or radiation therapy to treat ductal carcinoma in situ. Using this test we can select low-risk patients who can benefit from hormone therapy, avoiding chemotherapy and its side effects.
Interpretation of results: the low recurrence score (RS<18) means that the tumor is not so aggressive and has no benefit from chemotherapy, but is still very sensitive to hormonal treatment. For Intermediate Recurrence Score (18≤RS<31), the treating physician also considers other clinical-pathological parameters in addition to the outcome of the OncotypeDX analysis in order to decide on the treatment plan. These parameters may be the age of the patient or the grade of the tumor. High Recurrence Score (RS≥31) means that the tumor is more aggressive and requires adjuvant chemotherapy.
Oncotype test is included in all major breast cancer treatment guidelines: National Comprehensive Cancer Network (NCCN), American Society of Clinical Oncology (ASCO), the St. Gallen Consensus panel, National Institute for Health Care Excellence (NICE), and European Society for Medical Oncology (ESMO)(12).
Genetic testing in colon cancer
All Ras testing
Mutated Kras, Nras, Hras and BRAF represent predictive factors.
KRAS and NRAS genes are part of the same family. KRAS (exon 2) is mutated in about 40 percent of colon cancer cases. Approximately 10% of patients have mutations in other regions of the KRAS gene (exons 3 and 4) and the NRAS gene (exons 2, 3 and 4). Panitumumab and cetuximab are anti-EGFR monoclonal antibodies, which are attached to EGFR and blocks the proliferation and survival of cells that express EGFR (EGFR is overexpressed in colon cancer). The NCCN guideline specifies that for cases with wild type RAS gene type, should be taken into consideration BRAF testing. BRAF mutations reflect a poor prognostic and a poor response to therapy. The meaning of the presence of mutated Ras genes is the resistance to anti-EGFR target therapy.
The Oncotype DX Colon
Oncotype Recurrence Score quantifies recurrence risk in stage II and stage III colon cancer. This enables an individualized approach to treatment planning. The Oncotype DX test measures a group of cancer genes in the tumor. The results have predictive and prognostic implications. Approximately 28% of patients with colon cancer present with stage II disease, while 38% present with stage III disease. The test reflects the recurrence risk and can suggest adjuvant chemotherapy and its side effects can be avoided in low-risk patients. In stage II patients with T3 tumors, the Colon Recurrence Score result informs whether additional therapy should be considered as adjuvant condition. In stage III A/B patients, the Colon Recurrence Score result informs whether the absolute benefit of oxalipaltin based chemotherapy.
Microsatellite instability (MSI)
MSI is the condition of genetic errors that results from impaired DNA mismatch repair. The presence of MSI is evidence that DNA mismatch repair genes are not functioning normally. MMR corrects errors that spontaneously occur during DNA replication, such as single base mismatches or short insertions and deletions. Cells with abnormally MMR are unable to correct errors that occur during DNA replication and consequently accumulate errors. Polymerase chain reaction-based assays can provide evidence for the presence of MSI. High microsatellite instability is a feature that occurs in 90% of cases of hereditary Lynch syndrome. This is a prognostic test for patients with stage II which can identify the patients at high risk of recurrence. Also, the test identifies patients eligible for the Oncotype DX Colon test. It can be a predictive factor indicating the response to 5FU therapy.
Patients with high microsatellite instability (MSI-H) have a tumor less aggressive compared with patients with stable or unstable microsatellite instability of low degree. Patients with stage II colon cancer have a low recurrence risk, less than 5%. In these patients, chemotherapy can be avoided. 5-Fluorouracil-based chemotherapy has no benefit in these patients(9).
Genetic testing in lung cancer
EGFR mutations in non-small-cell lung cancer (NSCLC)
Approximately 10-16% of patients with NSCLC have tumor-associated EGFR. These mutations occurs within EGFR exons 18-21 (point mutations and deletions), which encode a portion of the EGFR kinase domain. The presence of mutations is a predictive factor for response to tyrosin-kinase inhibitors (erlotinib/gefitinib). The detection of EGFR mutations is performed by PCR, and then through DNA sequencing and comparison with reference sequences.
The resistance to TKI occurs following the T790M mutation on exon 20, which can also be detected by genetic testing.
EML4-ALK fusion
EML4-ALK positive lung cancer is a medical term that refers to a primary malignant lung tumor whose cells contain a characteristic abnormal configuration of DNA wherein the echinoderm microtubule-associated protein-like 4(EML4) gene is fused to the anaplastic lymphoma kinase (ALK) gene. This abnormal gene fusion leads to the production of a protein (EML4-ALK) that appears, in many cases, to promote and maintain the malignant behavior of the cancer cells. The transforming EML4-ALK fusion gene was first reported in non-small-cell lung carcinoma in 2-15% of cases. It is a predictive factor for response to tyrosine-kinase inhibitor crizotinib.
Predict DX Lung Cancer
Predict DX Lung Cancer is a test study of multiple genes, allowing physicians and patients taking a more individualized treatment decision. This multi-gene test offers individualized information about the treatment of patients through both modern targeted treatment, but also through targeted chemotherapeutic agents. DX analyzes gene mutations for EGFR-targeted treatment response and the expression of BRCA1, ERCC1, RRM1 and TS for resistance to chemotherapeutic agents, as well as specific gene fusion EML4/ALK(13).
Genetic testing in melanoma
BRAF is a human gene that encodes a protein called B-Raf. The gene is also referred to as proto-oncogene B-Raf and v-Raf murine sarcoma viral oncogene homolog B, while the protein is more formally known as serine/threonine-protein kinase B-Raf. The B-Raf protein is involved in sending signals inside cells which are involved in directing cell growth. In 2002, it was shown to be mutated in some human cancers. BRAF gene mutations are found in many cancers, such as malignant melanoma (27-40%), colon cancer (5-22%) and other cancers. The most common mutation of the BRAF gene is the V600E mutation, which accounts for approximately 86% of the BRAF mutations in cancerous tissue. Patients with metastatic malignant melanoma who are carriers of the V600E mutation on the BRAF gene may benefit from vemurafenib/dabrafenib targeted treatment.
Genetic testing in brain tumors
MGMT (D-methyl-guanine-DNA methyl transferase) is a gene that produces a DNA repairing enzyme which blocks the death of cancer cells by alkylating agents. High levels of this enzyme on tumors can cause resistance to treatment with alkylating agents such as procarbazine, streptozotocin and temozolomide. The activity of MGMT is controlled by its promoter. Methylation of the promoter suppresses gene expression and thus cells do not produce MGMT. This lack of enzyme may increase the sensitivity of malignant tumors to alkylating agents (such as temozolomide). Approximately 30% of gliomas have no MGMT. These findings represent a prognostic and predictive marker for patients with gliomas. Therefore, the correct treatment can be based on genome analysis(14).
Genetic testing in ovarian cancer
The BRCA genes are normally involved in the pathway of DNA repair, therefore mutations in these genes can block that pathway. By blocking the PARP (poly ADP ribose-polymerase) pathway, PARP inhibitors like olaparib or niraparib make it very hard for tumor cells with a mutated BRCA gene to repair damage DNA, which often leads to the death of these cells(15).
Conclusions
At the moment, genetic tests have become an important and necessary part of the diagnosis and treatment algorithm of cancer. Genetic testing becomes mandatory in many cancers for the correct orientation of treatment. Genetic tests have a prognostic and predictive role, depending on the outcome of the treatment and on the prognosis group in which the patient is framed. Therefore, we can speak at this moment about customised therapies tailored to each individual patient, depending on molecular biology of the tumor, prognostic factors and predictive factors. Targeted therapies are selected according to the mutations identified at the tumor level, which offer a major advantage in terms of response rate, time to disease progression and median survival.
Also, the benefit of adjuvant therapies or certain types of cytotoxic agents may be estimated by preliminary genetic testing, thereby avoiding additional toxicity in patients with good prognosis at low risk of recurrence.
Bibliografie
2. Weinstein IB, Joe A. Oncogene addiction. Cancer Res. 2008 May 1; 68(9):3077-80.
3. Heidi Chial, Chial H. Proto-oncogenes to Oncogenes to Cancer. 2008. Nature Education 1(1):33.
4. Zhang Z, Li M, Rayburn ER, Hill DL, Zhang R, Wang H. Oncogenes as novel targets for cancer therapy (part I): growth factors and protein tyrosine kinases. Am J Pharmacogenomics. 2005; 5(3):173-90.
5. Workman P. The opportunities and challenges of personalized genome-based molecular therapies for cancer: targets, technologies, and molecular chaperones. Cancer Chemother Pharmacol. 2003 Jul; 52 Suppl 1:S45-56. Epub 2003, Jun 18.
6. American Society of Clinical Oncology policy statement update: genetic testing for cancer susceptibility. J Clin Oncol. 2003 Jun 15; 21(12):2397-406. Epub 2003 Apr 11.
7. Peters JA, Stopfer JE. Role of the genetic counselor in familial cancer. Oncology (Williston Park). 1996 Feb; 10(2):159-66, 175; discussion 176-6, 178.
8. Hampel H, Frankel WL, Martin E. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008 Dec 10; 26(35):5783-8. doi: 10.1200/JCO.2008.17.5950. Epub 2008 Sep 22.
9. Buza N, Ziai J, Hui P. Mismatch repair deficiency testing in clinical practice. Expert Rev Mol Diagn. 2016; 16(5):591-604. doi: 10.1586/14737159.2016.1156533. Epub 2016 Mar 10.
10. Peltomaki P. Epigenetic mechanisms in the pathogenesis of Lynch syndrome. Clin Genet. 2014 May; 85(5):403-12. doi: 10.1111/cge.12349. Epub 2014 Feb 17.
11. Bert Vogelstein, Kenneth W Kinzler. Cancer genes and the pathways they control. Nature Medicine 10, 2004, 789–99.
12. Tutt A, Ashworth A. Can genetic testing guide treatment in breast cancer? Eur J Cancer. 2008 Dec; 44(18):2774-80. doi: 10.1016/j.ejca.2008.10.009. Epub 2008 Nov 20.
13. Mark G. Kris, Bruce. E. Johnnson, Lynne D. Barry, et al. Using Multiplexed Assays of Oncogenic Drivers in Lung Cancers to Select Targeted Drugs. JAMA. 2014; 311(19):1998-2006. doi:10.1001/jama.2014.3741.
14. Workman P. New drug targets for genomic cancer therapy: successes, limitations, opportunities and future challenges. Curr Cancer Drug Targets. 2001 May; 1(1):33-47.
15. Edlich RF, Winters KL, Lin KY. Breast cancer and ovarian cancer genetics. J Long Term Eff Med Implants. 2005; 15(5):533-45.
Articole din ediţiile anterioare
Mai există un rol pentru nefrectomia citoreducţională în era terapiilor ţintite şi a imunoterapiei?
Renal cell carcinoma (RCC), the most frequent type of kidney cancer, responsible for 5% of oncological diagnosis in men and for 3% in women, repres...