Nearly half of all cancer patients unavoidably experience brain metastases (BM) somewhere during their disease course. Current treatment approaches include neurosurgery, whole-brain radiotherapy (WBRT), stereotactic radiosurgery (SRS), chemotherapy, and targeted agents which may be utilized either individually or as any combination, depending on the anticipated patient’s prognosis. Radiotherapy, directed to whole-brain contents or exclusively the BMs, is the cornerstone of palliative management of patients with BM. This present review manuscript will particularly focus on the use of two well-established effective palliative radiotherapy modalities and their comparative effectiveness, namely the WBRT and SRS.
RADIOTERAPIA METASTAZELOR
Radioterapia în managementul metastazelor cerebrale
Radiotherapy in management of brain metastases
First published: 24 martie 2017
Editorial Group: MEDICHUB MEDIA
Abstract
Rezumat
Aproape jumătate din pacienții oncologici vor prezenta metastaze cerebrale (MC) pe parcursul evoluției bolii. Abordările terapeutice actuale includ neurochirurgia, radioterapia cerebrală totală (RCT), radiochirurgia stereotactică (RCS), chimioterapia și terapii țintite, care pot fi utilizate atât individual, cât și în combinație, depinzând de prognosticul pacientului. Radioterapia, fie că este cerebrală totală sau la nivelul metastazelor cerebrale, este baza tratamentului paleativ al pacienților cu MC.
Acest articol se va concentra pe utilizarea a două modalități eficiente de radioterapie paleativă - RCT și RCS, comparând rezultatele obținute cu fiecare dintre ele.
Introduction
Brain metastases, which are up to 10-fold more common than primary brain tumors, are experienced in approximately 40% of all cancer patients(1,2). Moreover, these rates rise up to 50% in malignant melanomas and 64% in lung cancer patients(3,4). Overall, there is a tendency for increment of BM incidence in cancer patients that is mainly associated with the implementation of more sophisticated imaging modalities and prolonged survival of the patients with more effective treatment of the primary cancer sites(5).Management of this one of the most regretful complication of cancer involves individual or as combined use of neurosurgery, whole-brain radiotherapy (WBRT), stereotactic radiosurgery (SRS), chemotherapy, and targeted agents with great attention paid for the prognostic factors and patients’ needs.
The main purpose of this review is to particularly focus on radiotherapeutic management of BM, with a specific emphasis on WBRT and stereotactic radiosurgery (SRS).
Biologic basis of BM
Biologically, BM development is a multistep process and involves both the malignant tumor cells and tumor microenvironment(6). In order to form BM, tumor cell(s) need to at first escape from the primary tumor mass and enter the blood stream, usually through the arterial system. Then, tumor cells mainly aggregate at the branching points of the cerebral vessels, which are trailed by the transmigration and invasion processes through the blood-brain barrier (BBB) by utilizing various endothelial surface molecules.Following passage of the BBB, tumor cells begin to interact with extracellular lattice, astrocytes, other stromal cells via paracrine signaling molecules like cytokines and growth factors. This interaction is one central point that is fundamental for tumor cell survival at the metastatic site.
Further growth and invasiveness of BM in brain is performed with the aid of some enzymes, such as heparanase and matrix metalloproteinases(7)
Prognostic factors
For patients, BM is probably the most regretful complication of the cancer, as it may emerge at any time during the course of treatment and as it has the greatest negative impact on quality of life measures and overall life span, with an expected median survival of 1-2 months with best supportive care and only 4-7 months even with aggressive multimodality treatment(8).Management of BM involves surgery, whole brain radiotherapy (WBRT), stereotactic radiosurgery (SRS), chemotherapy, and targeted agents individually or as any combination of them, that is determined by the prognostic factors.
Although presence of BM is usually connected with limited survival expectation, yet it is vital to perceive that majority of deaths are still associated with extracranial disease progression rather than the BM(9).
Considering the fact that patients may present with any disease and general health condition, the most crucial step of determination of most proper palliative treatment is to uncover the prognostic condition of the BM patients. Such a prognostic stratification will without a doubt serve gainful in distinction of the subgroup of patients who are anticipated to benefit significantly from more aggressive multimodality treatments than those who are less likely.
Additionally, poor prognostic patients can be saved from unnecessary toxic treatment maneuvers with this approach.
The universally accepted patient and tumor related prognostic factors include: performance status, age, number, site and size of BM, primary tumor histology, primary tumor status (controlled versus uncontrolled), presence/absence of extra-cranial metastases, and the interval between the primary cancer diagnosis and emergence of BM (synchronous versus metachronous)(5,10-12).
Based on these factors, various prognostic categorizations have been introduced for patients with BM who were fundamentally treated with WBRT(13).
In 1997, utilizing the recursive partitioning analysis (RPA) methodology in 1200 BM patients previously enrolled on three Radiation Therapy Oncology Group (RTOG) studies and received WBRT, Gaspar et al.(14) proposed a scoring system which included the KPS, age, primary tumor control status, and the status of extracranial metastases as the main determinants of prognosis. As per this categorization, patients with KPS≥70, age <65 years, controlled primary, and no extracranial systemic metastasis were incorporated into RPA class I and had the best prognosis (median: 7.1 months).
Patients with KPS<70 were classified as class III, with the worst survival (median: 2.3 months), while remaining patients were classified as RPA II with an intermediate survival outcome (median: 4.2 months). Though RTOG RPA classification is the most established and frequently used prognostic tool for stratification of BM patients, yet it has certain critical restrictions, such as the definition of lower KPS bound at 70, lack of the number of BM, and non-uniform WBRT doses utilized in the reference trials(13).
Moreover, uneven quantitative dispersion of patients in the gatherings, as most were RPA class II, may further limit the feasible use of RPA grouping in routine clinical practice(15).
Also, in spite of the fact that the disease characteristics and outcomes of such patients may vary widely, all patients with KPS<70 were incorporated into RPA class III regardless of the other potential variables which may modify survival outcomes in a significant manner. Supporting these adverse comments, by analyzing the outcomes of 113 patients, Nieder et al.(16) reported that there was no survival difference between patients in class II and III (3.6 vs. 4.2 months) after 30 Gy WBRT.
Another prognostic classification, score index for radiosurgery (SIR), incorporates the number of BM, volume of the largest BM, location of BM and post radiosurgery WBRT notwithstanding the variables of RPA classification(10).
The SIR was proposed to be more reliable than RPA in predicting survival after SRS, which was later validated with further studies in patients subjected to surgery with/without WBRT.
On the other hand, in the light of the fact that SIR was generated depending on only the outcomes of 65 SRS patients, it might be less illustrative for the dominant part of BM patients than the RPA classification(17). Probably because of these facts and requirement for detailed work up for assessment of the systemic disease, the SIR has gained a limited acceptance in the routine oncology clinics.
Management of BM
Traditionally, BM management strategies are usually divided into two, namely symptomatic and therapeutic. However, almost all strategies are interconnected. For instance, even when either one of surgery, SRS or WBRT is decided to be used as the sole treatment choice it may not be conceivable to stay away from use of corticosteroids or anticonvulsants so as to avoid or treat the edema, mass effect, convulsions, and/or side effects of the treatment.While the common purpose of the both managements is to improve quality of life and neurological status of the patient, it might likewise be conceivable to enhance the survival with appropriately selected treatment maneuver in some certain patients population, such as the use of SRS in oligometastatic BM patients with primary non-small cell lung carcinoma(18).
Whole Brain Radiotherapy (WBRT)
WBRT is the most commonly used symptom relieving treatment option for patients with BM; especially those presenting with large and/or multiple BM, eloquent tumor locations, or rejecting neurosurgery(19). Additionally, postoperative WBRT has been utilized postoperatively as an adjunct complementary treatment against residual or microscopic disease, with some reports of prolonged median survival rates ranging between 1 to 2 years(20,21).
However, the median survival or symptom control has not shown to be further enhanced by variations in total doses, timing, and fractionation of WBRT as indicated by a meta-analysis of the RTOG trials(22).
Currently, 30 Gy (10 fractions) is the most popular treatment regime for WBRT of BM patients, considering the risk for higher neurotoxicity with daily fraction doses >3 Gy. A three dimensional conformal plan is shown in Figure 1.
Various strategies have been tested in order to improve the outcomes of BM patients undergoing WBRT. One such strategy is the concurrent use of radiation sensitizers. Although the agents such as misonidazole, bromodeoxyuridine, lonidamine, nimustine, fluorouracil have failed to improve local control or overall survival in randomized controlled trials, some agents like motexafin-gadolinium (MGd) and efaproxaril appeared to be promising in some certain groups of BM patients(5).
MGd is a tumor selective metallaporphyrin redox modulator that mediates oxidation of several intracellular metabolites, such as nicotinamide adenine dinucleotide phosphate, ascorbate and glutation, and thereby produces reactive oxygen species which results in enhanced tumor specific cytotoxicity by blockage of tumor cell damage repair capacity in absence of redox products(23).
Mehta et al. utilized MGd in a randomized phase III study incorporating 401 patients with BM and demonstrated that time to neurologic progression was specifically improved in the subgroup of 251 non-small cell lung cancer patients(24).
Efaproxaril is an agent that impedes the oxygen binding affinity of hemoglobin by a noncovalent binding process, and therefore, shifts the oxygen dissociation curve to the left which results in increased tumoral oxygenation(23).
In a phase III randomized trial of 538 patients with BM, eligible patients were randomized into one of WBRT plus supplemental oxygen in presence or absence of efaproxaril arms.
Results of this study was encouraging in the subgroup of 115 patients with breast cancer in terms of median survival durations (8.7 vs. 4.6 months, p= 0.061)(25).
Chemotherapy is a limitedly used upfront treatment modality in management of BM patients which might rather be preferred as a salvage option for WBRT and/or SRS relapses(26).
Several agents such as tegafur, teniposide and nitrosureas have been utilized in the treatment of BM in combination with WBRT; however, the observed response rates were limited with no survival benefit and unfortunately in expense of increased toxicity rates(27,28).
Temozolomide (TMZ), an oral alkylating agent with almost perfect central nerve system penetration, has been utilized in the patients with BM of solid cancers, including lung cancer, with reported objective tumor responses and neurologic improvements(29,30).
Antonadou et al. showed a 96% objective response rate (38% complete) in 24 BM patients undergoing WBRT and TMZ combination compared to 66% responders in 21 patients treated with WBRT only.
However, this enhanced response rate with TMZ did not translate into a survival advantage (8.6 months versus 7 months, p= 0.447)(30). Therefore, current standard management of BM does not include TMZ.
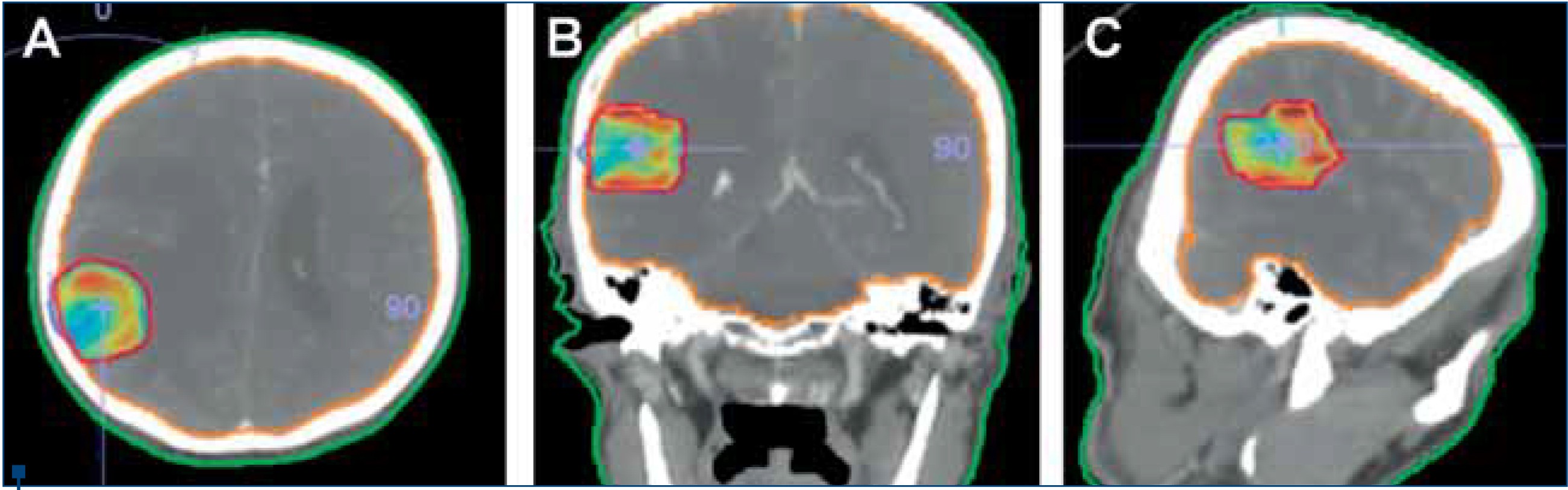
(A) Axial, (B) Coronal, (C) Sagittal views
Stereotactic Radiosurgery (SRS)
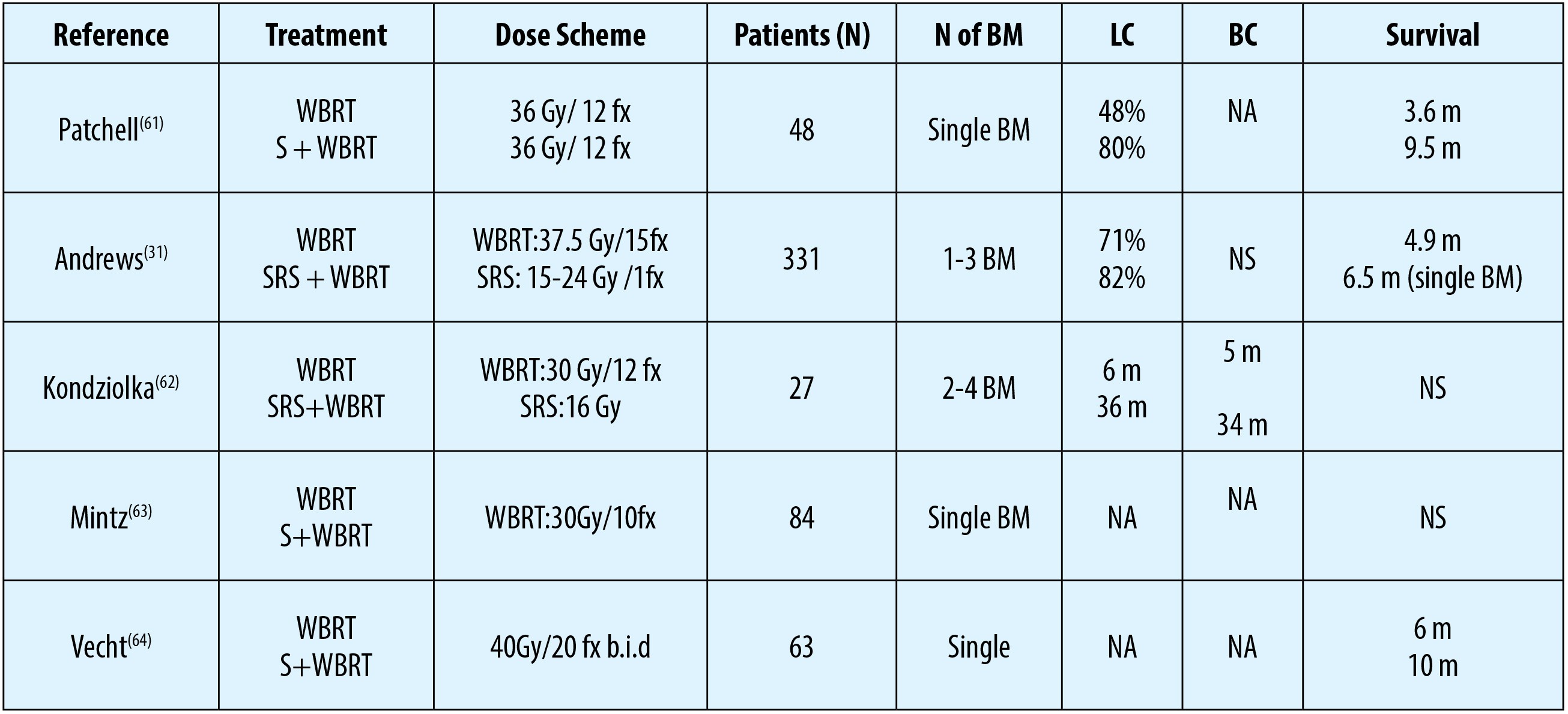
Although WBRT is the most commonly utilized palliative option in the management of BM, local control rates of treated lesion(s) are unsatisfactory with WBRT alone(31,32). As more than half of the BM manifest as a single solitary lesion, WBRT is also denounced for possibly being overtreatment WBRT with significant neurocognitive side effects(33,34). Because of these worries SRS has gained significant popularity in the most recent decades either as a single treatment choice or complementary/ salvage modality after WBRT or WBRT relapses(35). In this respect, WBRT alone has been compared with WBRT combined with local therapies, as depicted in Table 1. SRS with its >80% infield tumor control rates is a very efficient therapeutic option for suitable targets and has the additional advantages of being applicable in a single session without requirement for hospitalization, bringing about no noteworthy deferrals in systemic treatments like chemotherapy.
Moreover, because of its dosimetric superiority and distinctive radiobiological features, toxicity and radioresistance concerns are of SRS are of relatively lower importance compared to WBRT(36). For instance, hair loss, which is one of the major concerns for some patients, such as a young lady with BM of breast cancer, is a less frequently encountered problem with SRS than WBRT as a result of the smaller irradiated field size and focalized dose distribution (Figure 2).
Fatigue, as an often complaint of WBRT, may likewise be experienced less frequently with SRS. All the aforementioned advantages of SRS are provided by utilization of multiple convergent narrow beams to deliver high dose focal irradiation in a single fraction by using multiple cobalt-60 sources, linear accelerators or cyclotrons(37,38).
SRS alone or SRS after WBRT are confirmed standard treatment approaches in the management of patients with single BM (Table 2)(35,39,40). Similar with neurosurgery, SRS alone or in combination with WBRT has been exhibited to associate with prolonged overall survival, local control and also better neurologic status in these patients compared to WBRT alone(33,34).
However, SRS differs from neurosurgery by offering a chance of ablative treatment to those patients who are not appropriate candidates for neurosurgery due to various reasons.
Superiorities of SRS have brought the inquiry whether the omission of WBRT may be conceivable in management of patients with limited number of BM. Albeit such an approach may be beneficial in a select group of patients, prerequisites for close monitorization with monthly or bimonthly magnetic resonance imaging (MRI) and risk for unavoidable repeat SRS procedures for newly emerging BM, both increasing the total cost of overall treatment, should be carefully considered(23). Moreover, contrasted with SRS and WBRT combination, the risk for a plausibility of inferior survival outcomes with SRS alone in patients with controlled primary and no extracranial disease should be kept in mind, as it has been accentuated previously by various authors(41,42).
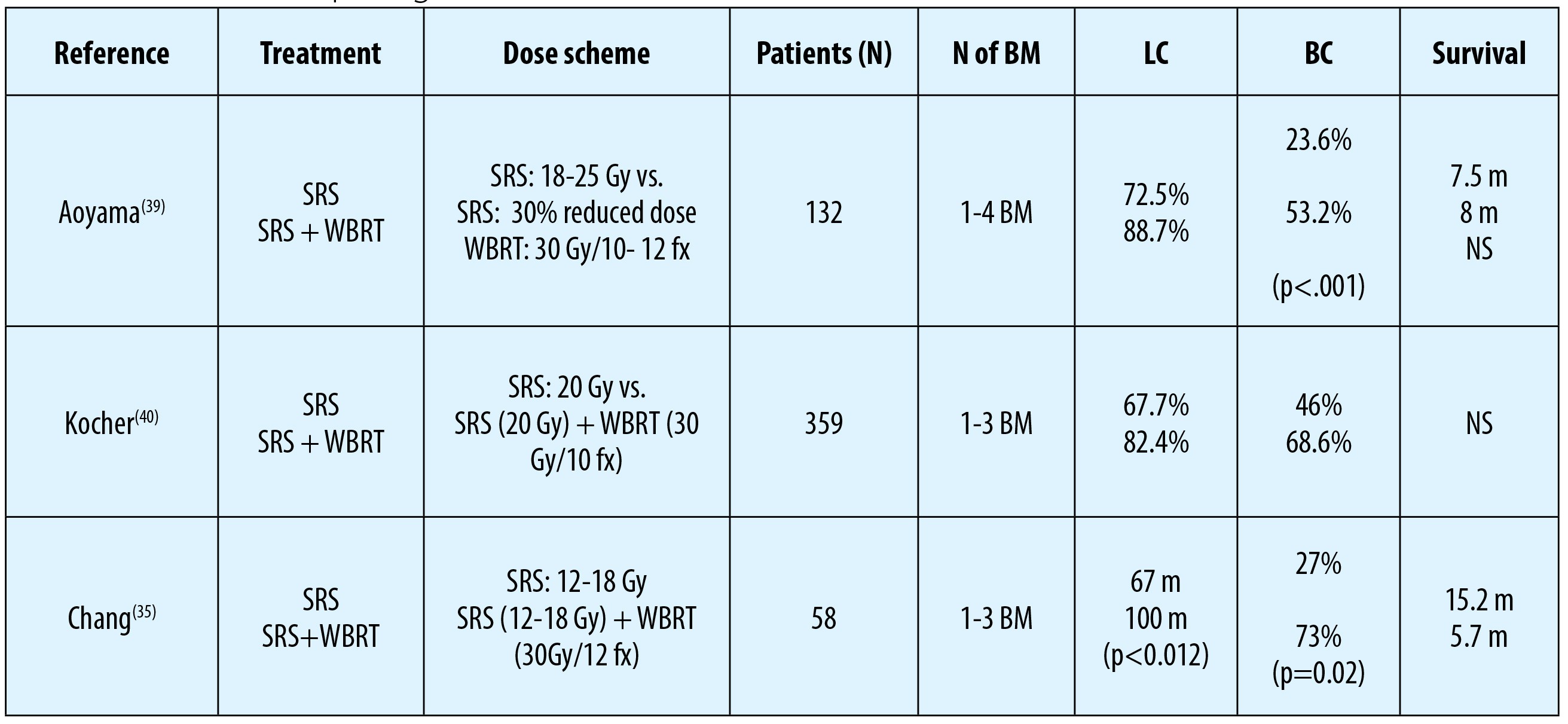
To date, three randomized controlled trials (RCT) have comparatively evaluated the efficacy of SRS alone against SRS with WBRT in patients with 1-4 BM(35,39,40). Although local- and distant brain control rates were reported to be better with the addition of WBRT, this distinction did not translate into a notable survival advantage in any study.
Furthermore, in the study by Chang et al., authors showed a survival advantage favoring SRS alone over SRS and WBRT combination in patients with 1-3 BM (p= 0.003)(35).
It is unfortunate to point out that the results of these RCTs ought to be interpreted with caution because of their insufficient design to explicitly concentrate on survival endpoints, such as significant imbalances between the study groups with regards to the prognostic factors and utilization of salvage WBRT in SRS alone cohorts(43,44).
There are also four meta-analyses investigating the effect of WBRT combined with SRS against SRS alone for patients with BM(45-48).
First meta-analysis was performed by Duan et al.(46) and cooperated four RCTs. Although 1-year survival rate was statistically not different between arms, SRS alone was related with better neurocognitive functions, while combined modality treatment was found to associate with local (OR=0.29, 95% CI: 0.17-0.49) and distant brain failures (OR=0.45, 95% CI: 0.28-0.71).
In the second meta-analysis, Hasan et al.(48) reported the results of meta-analysis of three RCTs including 389 patients with 1 to 4 BM.
According to 2 of these trials, combination treatment was superior than SRS alone in terms of 1-year local control (89% vs. 71%, p=0.001) and distant brain control (63.4% vs. 46%, p=0.001) rates with no significant impact on symptom control probability and survival outcomes.
Thirdly, the meta-analysis by Soon et al.(47) incorporated five RCTs of patients with 1-4 BM, and demonstrated a superior intracranial disease control rate at one year in combined arm, but again this difference did not translate into any prolongation of either overall or progression free-survival times.
In the fourth and most recent meta-analysis, by Sahgal et al.(45), results of three RCTs depicted a survival advantage for patients ≤50 years of age who were treated with SRS alone compared to their age-matched cohort treated with SRS and WBRT combination (P=0.04). Additionally omission of WBRT in this subgroup was not identified to relate with increased rates of distant brain relapses.
In patients >50 years of age, although there was no survival difference between the two treatment arms, the risk of distant BM after SRS alone was significantly higher (p=0.043).
Based on these results, the authors recommended SRS alone as the preferred treatment option targeting BM for patients≤50 years.
Another relatively new SRS methodology procedure includes its use for the treatment of postsurgical tumor cavity (TC-SRS).
This novel SRS strategy has emerged to combat against high tumor cavity failures (46-59%) after neurosurgical removal of the BM, and as a less neurotoxic alternative to WBRT(40,49,50).
In a recent systematic review of 14 studies incorporating 629 BM patients, Gans et al. reported excellent median survival (14 months) and 1-year local control rates (85%) for all studies combined(50).
Local control was correlated with the median volume treated with radiosurgery (r = -0.766, P <0.05) and with the rate of gross total resection (r = .728, P <0.03). Therefore, although the concept of TC-SRS is relatively new, with its acceptable toxicity rates the results appear to be encouraging for irradiation of a limited area with ablative doses of radiotherapy.
Brain irradiation and neurocognitive toxicity
The impact of WBRT on neurocognitive functions is unclear, but yet, one major concern about the usage of WBRT as a sole or adjunct treatment option in BM patients is its potential to impair the these functions, and therefore the related quality of life measures(51).In a study by Pinkham et al., authors demonstrated that WBRT altered Hopkins Verbal Learning Test Revised (HVLT-R) and Trail Making Test (TMT) part B scores with no influence on Mini-Mental State Examination (MMSE) score.
Verbal memory and fine motor functions were the commonest parameters to be impaired in this study(51).
Theoretically, restriction of the irradiated brain volume with local therapies like surgery and SRS may prove beneficial in preservation of neurocognitive functions without any scarification in tumor control rates. This theoretical advantage formed the basis for increased interest in SRS and avoidance of WBRT or hippocampal sparing WBRT for management of patients presenting with BM.
Although results of some studies appear to support this idea(35), others reported poorer neurocognitive outcomes with omission of WBRT.
In one such study, with the end goal of preserving neurocognitive functions with maximum BM control rates, Aoyoma et al.(39) evaluated neurocognition by using MMSE in patients with 1-4 BM randomized to one of WBRT plus SRS versus SRS alone arms.
The authors reported that the average interval between the initiation of treatment and any notable decline in neurocognitive functions was significantly longer in the WBRT + SRS than the SRS-alone group (16.5 vs. 7.6 months; p=0.05).
It is important to notify that the toxicities attributed exclusively to WBRT are also likely dependent on certain risk including the patient’s age, preceding or concomitant chemotherapy usage and other putative risk factors that are to date not exactly defined as such smoking, diabetes mellitus, radiation hypersensitivity syndromes, hypothalamohypophseal abnormalities and/or atherosclerosis.
Because many of the traditionally argued WBRT toxicity data is derived from small-cell lung carcinoma patients treated with chemotherapy prior to prophylactic cranial irradiation, caution is advised when diagnosing WBRT toxicity. This issue is critically important as the influence of chemotherapy on cognitive functions of many cancer patients remained underestimated for a long time, namely ‘chemo-fog’ or ‘chemobrain’, which negatively impacts upon the quality of life measures by alterations in attention, concentration, speed of information processing, verbal and visual memory, multitasking and ability to organize information(52-56).
Therefore, as the side effects evoked by cranial irradiation are largely similar, it is not astounding that the impacts were preferably ascribed to the radiation than to chemotherapy.
Notwithstanding, over a decade ago, it has been noted that patients receiving standard-dose adjuvant chemotherapy for breast cancer were associated with decreased cognitive memory and language functions as compared to matched control groups(57).
This information is of foremost significance for radiation oncologists considering the way that almost all toxicities following therapeutic WBRT are almost constantly ascribed to cranial irradiation by the other oncologic disciplines.
Deteriorations in neurocognitive functions may also be already present before the initiation of WBRT. This issue has been addressed in two key studies by Meyers et al.(58) and Komaki et al.(59).
In the Meyers’s phase III RCT comparing WBRT versus WBRT plus motexafin-gadolinium (MGd), the authors reported that 90.5% of all patients had maladies in neurocognitive functions at the time of BM diagnosis, of which 42% had impairments in at least four out of the eight domains(58).
In the second study by Komaki et al., small-cell lung cancer patients were asked to perform neuropsychological tests just before inception and completion of prophylactic cranial irradiation after combination chemotherapy and thoracic radiotherapy(59).
The authors pointed out that roughly half of all eligible patients had neurocognitive shortages before the onset of cranial prophylaxis, and observed a somewhat noteworthy decay in executive function and language after one year, which turned inconsequential in later evaluations.
These two excellent studies strongly emphasize the paramount importance of implementation of neurocognitive function tests prior to WBRT in order to reflect the actual impact of therapeutic WBRT on neurocognitive domains.
Moreover, the negative neurocognitive impact of progressive BM may further be ameliorated or even improved by WBRT in some patients groups with resultant enhancement in executive functions and fine motor co-ordination as neurologic deterioration is reported to directly relate with disease progression in the brain(51,60).
Conclusions
Approximately 40% of all cancer patients unavoidably experience BM somewhere during their treatment or follow-up.Management of this regretful complication of cancer involves neurosurgery, WBRT, SRS, chemotherapy, and targeted agents individually or as any combination of them, regarding the prognostic factors.
Although both the WBRT and SRS are established radiotherapy options for patients presenting with BM and SRS alone is associated with higher rates in brain relapses, recent RCTs and meta-analyses suggest SRS alone as the initial therapy for patients with 1 to 4 BM with a survival advantage specifically in patients ≤50 years of age. n
Bibliografie
1. Mehta MP, Tsao MN, Whelan TJ, et al: The American Society for Therapeutic Radiology and Oncology (ASTRO) evidence-based review of the role of radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys 63:37-46, 2005.
2. Shaffrey ME, Mut M, Asher AL, et al: Brain metastases. Curr Probl Surg 41:665-741, 2004.
3. Sorensen JB, Hansen HH, Hansen M, et al: Brain metastases in adenocarcinoma of the lung: frequency, risk groups, and prognosis. J Clin Oncol 6:1474-80, 1988.
4. Amer MH, Al-Sarraf M, Baker LH, et al: Malignant melanoma and central nervous system metastases: incidence, diagnosis, treatment and survival. Cancer 42:660-8, 1978.
5. Eichler AF, Loeffler JS: Multidisciplinary management of brain metastases. Oncologist 12:884-98, 2007.
6. Arvatz G, Shafat I, Levy-Adam F, et al: The heparanase system and tumor metastasis: is heparanase the seed and soil? Cancer Metastasis Rev 30:253-68, 2011.
7. Ridgway LD, Wetzel MD, Marchetti D: Modulation of GEF-H1 induced signaling by heparanase in brain metastatic melanoma cells. J Cell Biochem 111:1299-309, 2010.
8. Berk L: An overview of radiotherapy trials for the treatment of brain metastases. Oncology (Williston Park) 9:1205-12; discussion 1212-6, 1219, 1995.
9. Gondi V, Tome WA, Marsh J, et al: Estimated risk of perihippocampal disease progression after hippocampal avoidance during whole-brain radiotherapy: safety profile for RTOG 0933. Radiother Oncol 95:327-31, 2010.
10. Weltman E, Salvajoli JV, Brandt RA, et al: Radiosurgery for brain metastases: a score index for predicting prognosis. Int J Radiat Oncol Biol Phys 46:1155-61, 2000.
11. Sperduto PW, Kased N, Roberge D, et al: Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 30:419-25, 2012.
12. Abrahams JM, Torchia M, Putt M, et al: Risk factors affecting survival after brain metastases from non-small cell lung carcinoma: a follow-up study of 70 patients. J Neurosurg 95:595-600, 2001.
13. Venur VA, Ahluwalia MS: Prognostic scores for brain metastasis patients: use in clinical practice and trial design. Chin Clin Oncol 4:18, 2015.
14. Gaspar L, Scott C, Rotman M, et al: Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 37:745-51, 1997.
15. Park JY, Moon KS, Lee KH, et al: Gamma knife radiosurgery for elderly patients with brain metastases: evaluation of scoring systems that predict survival. BMC Cancer 15:54, 2015.
16. Nieder C, Andratschke N, Grosu AL, et al: Recursive partitioning analysis (RPA) class does not predict survival in patients with four or more brain metastases. Strahlenther Onkol 179:16-20, 2003.
17. Rades D, Dziggel L, Haatanen T, et al: Scoring systems to estimate intracerebral control and survival rates of patients irradiated for brain metastases. Int J Radiat Oncol Biol Phys 80:1122-7, 2011.
18. Topkan E, Parlak C, Kotek A, et al: Impact of prophylactic cranial irradiation timing on brain relapse rates in patients with stage IIIB non-small-cell lung carcinoma treated with two different chemoradiotherapy regimens. Int J Radiat Oncol Biol Phys 83:1264-71, 2012.
19. Coia LR: The role of radiation therapy in the treatment of brain metastases. Int J Radiat Oncol Biol Phys 23:229-38, 1992.
20. Teicher BA, Waxman DJ, Holden SA, et al: Evidence for enzymatic activation and oxygen involvement in cytotoxicity and antitumor activity of N,N’,N’’-triethylenethiophosphoramide. Cancer Res 49: 4996-5001, 1989.
21. Holden SA, Teicher BA, Ha C, et al: Enhancement by perflusion emulsion (Oxygent) and carbogen breathing of the tumor growth delay of the FSaIIC fibrosarcoma after treatment with antitumor alkylating agents. Biomater Artif Cells Immobilization Biotechnol 20:895-8, 1992.
22. Tsao MN, Lloyd N, Wong R, et al: Whole brain radiotherapy for the treatment of multiple brain metastases. Cochrane Database Syst Rev: CD003869, 2006.
23. Khuntia D, Brown P, Li J, et al: Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol 24:1295-304, 2006.
24. Mehta MP, Rodrigus P, Terhaard CH, et al: Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole-brain radiation therapy in brain metastases. J Clin Oncol 21:2529-36, 2003.
25. Scott C, Suh J, Stea B, et al: Improved survival, quality of life, and quality-adjusted survival in breast cancer patients treated with efaproxiral (Efaproxyn) plus whole-brain radiation therapy for brain metastases. Am J Clin Oncol 30:580-7, 2007.
26. Quantin X, Khial F, Reme-Saumon M, et al: Concomitant brain radiotherapy and vinorelbine-ifosfamide-cisplatin chemotherapy in brain metastases of non-small cell lung cancer. Lung Cancer 26:35-9, 1999.
27. Mornex F, Thomas L, Mohr P, et al: A prospective randomized multicentre phase III trial of fotemustine plus whole brain irradiation versus fotemustine alone in cerebral metastases of malignant melanoma. Melanoma Res 13:97-103, 2003.
28. Ushio Y, Arita N, Hayakawa T, et al: Chemotherapy of brain metastases from lung carcinoma: a controlled randomized study. Neurosurgery 28:201-5, 1991.
29. Christodoulou C, Bafaloukos D, Kosmidis P, et al: Phase II study of temozolomide in heavily pretreated cancer patients with brain metastases. Ann Oncol 12:249-54, 2001.
30. Antonadou D, Paraskevaidis M, Sarris G, et al: Phase II randomized trial of temozolomide and concurrent radiotherapy in patients with brain metastases. J Clin Oncol 20:3644-50, 2002.
31. Andrews DW, Scott CB, Sperduto PW, et al: Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 363:1665-72, 2004.
32. Noordijk EM, Vecht CJ, Haaxma-Reiche H, et al: The choice of treatment of single brain metastasis should be based on extracranial tumor activity and age. Int J Radiat Oncol Biol Phys 29:711-7, 1994.
33. Fabi A, Felici A, Metro G, et al: Brain metastases from solid tumors: disease outcome according to type of treatment and therapeutic resources of the treating center. J Exp Clin Cancer Res 30:10, 2011.
34. Nussbaum ES, Djalilian HR, Cho KH, et al: Brain metastases. Histology, multiplicity, surgery, and survival. Cancer 78:1781-8, 1996.
35. Chang EL, Wefel JS, Hess KR, et al: Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 10:1037-44, 2009.
36. Yamamoto M, Serizawa T, Shuto T, et al: Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol 15:387-95, 2014.
37. Shaw E, Scott C, Souhami L, et al: Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys 47:291-8, 2000.
38. Shehata MK, Young B, Reid B, et al: Stereotatic radiosurgery of 468 brain metastases < or =2 cm: implications for SRS dose and whole brain radiation therapy. Int J Radiat Oncol Biol Phys 59:87-93, 2004.
39. Aoyama H, Shirato H, Tago M, et al: Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 295:2483-91, 2006.
40. Kocher M, Soffietti R, Abacioglu U, et al: Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol 29:134-41, 2011.
41. Pirzkall A, Debus J, Lohr F, et al: Radiosurgery alone or in combination with whole-brain radiotherapy for brain metastases. J Clin Oncol 16:3563-9, 1998.
42. Smalley SR, Laws ER, Jr., O’Fallon JR, et al: Resection for solitary brain metastasis. Role of adjuvant radiation and prognostic variables in 229 patients. J Neurosurg 77:531-40, 1992.
43. Knisely JP: Focused attention on brain metastases. Lancet Oncol 10:1024, 2009.
44. Weiss SE, Kelly PJ: Neurocognitive function after WBRT plus SRS or SRS alone. Lancet Oncol 11:220-1, 2010.
45. Sahgal A, Aoyama H, Kocher M, et al: Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: individual patient data meta-analysis. Int J Radiat Oncol Biol Phys 91:710-7, 2015.
46. Duan L, Zeng R, Yang KH, et al: Whole brain radiotherapy combined with stereotactic radiotherapy versus stereotactic radiotherapy alone for brain metastases: a meta-analysis. Asian Pac J Cancer Prev 15:911-5, 2014.
47. Soon YY, Tham IW, Lim KH, et al: Surgery or radiosurgery plus whole brain radiotherapy versus surgery or radiosurgery alone for brain metastases. Cochrane Database Syst Rev 3:CD009454, 2014.
48. Hasan S, Shah AH, Bregy A, et al: The role of whole-brain radiation therapy after stereotactic radiation surgery for brain metastases. Pract Radiat Oncol 4:306-15, 2014.
49. Roberge D, Parney I, Brown PD: Radiosurgery to the postoperative surgical cavity: who needs evidence? Int J Radiat Oncol Biol Phys 83:486-93, 2012.
50. Gans JH, Raper DM, Shah AH, et al: The role of radiosurgery to the tumor bed after resection of brain metastases. Neurosurgery 72:317-25; discussion 325-6, 2013.
51. Pinkham MB, Sanghera P, Wall GK, et al: Neurocognitive Effects Following Cranial Irradiation for Brain Metastases. Clin Oncol (R Coll Radiol) 27:630-9, 2015.
52. Vardy J, Tannock I: Cognitive function after chemotherapy in adults with solid tumours. Crit Rev Oncol Hematol 63:183-202, 2007.
53. van Dam FS, Schagen SB, Muller MJ, et al: Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: high-dose versus standard-dose chemotherapy. J Natl Cancer Inst 90:210-8, 1998.
54. Schagen SB, van Dam FS, Muller MJ, et al: Cognitive deficits after postoperative adjuvant chemotherapy for breast carcinoma. Cancer 85:640-50, 1999.
55. Brezden CB, Phillips KA, Abdolell M, et al: Cognitive function in breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol 18:2695-701, 2000.
56. Ganz PA, Desmond KA, Leedham B, et al: Quality of life in long-term, disease-free survivors of breast cancer: a follow-up study. J Natl Cancer Inst 94:39-49, 2002.
57. Wefel JS, Lenzi R, Theriault RL, et al: The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: results of a prospective, randomized, longitudinal trial. Cancer 100:2292-9, 2004.
58. Meyers CA, Smith JA, Bezjak A, et al: Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trial. J Clin Oncol 22:157-65, 2004.
59. Komaki R, Meyers CA, Shin DM, et al: Evaluation of cognitive function in patients with limited small cell lung cancer prior to and shortly following prophylactic cranial irradiation. Int J Radiat Oncol Biol Phys 33:179-82, 1995.
60. Regine WF, Huhn JL, Patchell RA, et al: Risk of symptomatic brain tumor recurrence and neurologic deficit after radiosurgery alone in patients with newly diagnosed brain metastases: results and implications. Int J Radiat Oncol Biol Phys 52:333-8, 2002.
61. Patchell RA, Tibbs PA, Walsh JW, et al: A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 322:494-500, 1990.
62. Kondziolka D, Patel A, Lunsford LD, et al: Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys 45:427-34, 1999.
63. Mintz AH, Kestle J, Rathbone MP, et al: A randomized trial to assess the efficacy of surgery in addition to radiotherapy in patients with a single cerebral metastasis. Cancer 78:1470-6, 1996.
64. Vecht CJ, Haaxma-Reiche H, Noordijk EM, et al: Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol 33:583-90, 1993.
2. Shaffrey ME, Mut M, Asher AL, et al: Brain metastases. Curr Probl Surg 41:665-741, 2004.
3. Sorensen JB, Hansen HH, Hansen M, et al: Brain metastases in adenocarcinoma of the lung: frequency, risk groups, and prognosis. J Clin Oncol 6:1474-80, 1988.
4. Amer MH, Al-Sarraf M, Baker LH, et al: Malignant melanoma and central nervous system metastases: incidence, diagnosis, treatment and survival. Cancer 42:660-8, 1978.
5. Eichler AF, Loeffler JS: Multidisciplinary management of brain metastases. Oncologist 12:884-98, 2007.
6. Arvatz G, Shafat I, Levy-Adam F, et al: The heparanase system and tumor metastasis: is heparanase the seed and soil? Cancer Metastasis Rev 30:253-68, 2011.
7. Ridgway LD, Wetzel MD, Marchetti D: Modulation of GEF-H1 induced signaling by heparanase in brain metastatic melanoma cells. J Cell Biochem 111:1299-309, 2010.
8. Berk L: An overview of radiotherapy trials for the treatment of brain metastases. Oncology (Williston Park) 9:1205-12; discussion 1212-6, 1219, 1995.
9. Gondi V, Tome WA, Marsh J, et al: Estimated risk of perihippocampal disease progression after hippocampal avoidance during whole-brain radiotherapy: safety profile for RTOG 0933. Radiother Oncol 95:327-31, 2010.
10. Weltman E, Salvajoli JV, Brandt RA, et al: Radiosurgery for brain metastases: a score index for predicting prognosis. Int J Radiat Oncol Biol Phys 46:1155-61, 2000.
11. Sperduto PW, Kased N, Roberge D, et al: Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 30:419-25, 2012.
12. Abrahams JM, Torchia M, Putt M, et al: Risk factors affecting survival after brain metastases from non-small cell lung carcinoma: a follow-up study of 70 patients. J Neurosurg 95:595-600, 2001.
13. Venur VA, Ahluwalia MS: Prognostic scores for brain metastasis patients: use in clinical practice and trial design. Chin Clin Oncol 4:18, 2015.
14. Gaspar L, Scott C, Rotman M, et al: Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 37:745-51, 1997.
15. Park JY, Moon KS, Lee KH, et al: Gamma knife radiosurgery for elderly patients with brain metastases: evaluation of scoring systems that predict survival. BMC Cancer 15:54, 2015.
16. Nieder C, Andratschke N, Grosu AL, et al: Recursive partitioning analysis (RPA) class does not predict survival in patients with four or more brain metastases. Strahlenther Onkol 179:16-20, 2003.
17. Rades D, Dziggel L, Haatanen T, et al: Scoring systems to estimate intracerebral control and survival rates of patients irradiated for brain metastases. Int J Radiat Oncol Biol Phys 80:1122-7, 2011.
18. Topkan E, Parlak C, Kotek A, et al: Impact of prophylactic cranial irradiation timing on brain relapse rates in patients with stage IIIB non-small-cell lung carcinoma treated with two different chemoradiotherapy regimens. Int J Radiat Oncol Biol Phys 83:1264-71, 2012.
19. Coia LR: The role of radiation therapy in the treatment of brain metastases. Int J Radiat Oncol Biol Phys 23:229-38, 1992.
20. Teicher BA, Waxman DJ, Holden SA, et al: Evidence for enzymatic activation and oxygen involvement in cytotoxicity and antitumor activity of N,N’,N’’-triethylenethiophosphoramide. Cancer Res 49: 4996-5001, 1989.
21. Holden SA, Teicher BA, Ha C, et al: Enhancement by perflusion emulsion (Oxygent) and carbogen breathing of the tumor growth delay of the FSaIIC fibrosarcoma after treatment with antitumor alkylating agents. Biomater Artif Cells Immobilization Biotechnol 20:895-8, 1992.
22. Tsao MN, Lloyd N, Wong R, et al: Whole brain radiotherapy for the treatment of multiple brain metastases. Cochrane Database Syst Rev: CD003869, 2006.
23. Khuntia D, Brown P, Li J, et al: Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol 24:1295-304, 2006.
24. Mehta MP, Rodrigus P, Terhaard CH, et al: Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole-brain radiation therapy in brain metastases. J Clin Oncol 21:2529-36, 2003.
25. Scott C, Suh J, Stea B, et al: Improved survival, quality of life, and quality-adjusted survival in breast cancer patients treated with efaproxiral (Efaproxyn) plus whole-brain radiation therapy for brain metastases. Am J Clin Oncol 30:580-7, 2007.
26. Quantin X, Khial F, Reme-Saumon M, et al: Concomitant brain radiotherapy and vinorelbine-ifosfamide-cisplatin chemotherapy in brain metastases of non-small cell lung cancer. Lung Cancer 26:35-9, 1999.
27. Mornex F, Thomas L, Mohr P, et al: A prospective randomized multicentre phase III trial of fotemustine plus whole brain irradiation versus fotemustine alone in cerebral metastases of malignant melanoma. Melanoma Res 13:97-103, 2003.
28. Ushio Y, Arita N, Hayakawa T, et al: Chemotherapy of brain metastases from lung carcinoma: a controlled randomized study. Neurosurgery 28:201-5, 1991.
29. Christodoulou C, Bafaloukos D, Kosmidis P, et al: Phase II study of temozolomide in heavily pretreated cancer patients with brain metastases. Ann Oncol 12:249-54, 2001.
30. Antonadou D, Paraskevaidis M, Sarris G, et al: Phase II randomized trial of temozolomide and concurrent radiotherapy in patients with brain metastases. J Clin Oncol 20:3644-50, 2002.
31. Andrews DW, Scott CB, Sperduto PW, et al: Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 363:1665-72, 2004.
32. Noordijk EM, Vecht CJ, Haaxma-Reiche H, et al: The choice of treatment of single brain metastasis should be based on extracranial tumor activity and age. Int J Radiat Oncol Biol Phys 29:711-7, 1994.
33. Fabi A, Felici A, Metro G, et al: Brain metastases from solid tumors: disease outcome according to type of treatment and therapeutic resources of the treating center. J Exp Clin Cancer Res 30:10, 2011.
34. Nussbaum ES, Djalilian HR, Cho KH, et al: Brain metastases. Histology, multiplicity, surgery, and survival. Cancer 78:1781-8, 1996.
35. Chang EL, Wefel JS, Hess KR, et al: Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 10:1037-44, 2009.
36. Yamamoto M, Serizawa T, Shuto T, et al: Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol 15:387-95, 2014.
37. Shaw E, Scott C, Souhami L, et al: Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys 47:291-8, 2000.
38. Shehata MK, Young B, Reid B, et al: Stereotatic radiosurgery of 468 brain metastases < or =2 cm: implications for SRS dose and whole brain radiation therapy. Int J Radiat Oncol Biol Phys 59:87-93, 2004.
39. Aoyama H, Shirato H, Tago M, et al: Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 295:2483-91, 2006.
40. Kocher M, Soffietti R, Abacioglu U, et al: Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol 29:134-41, 2011.
41. Pirzkall A, Debus J, Lohr F, et al: Radiosurgery alone or in combination with whole-brain radiotherapy for brain metastases. J Clin Oncol 16:3563-9, 1998.
42. Smalley SR, Laws ER, Jr., O’Fallon JR, et al: Resection for solitary brain metastasis. Role of adjuvant radiation and prognostic variables in 229 patients. J Neurosurg 77:531-40, 1992.
43. Knisely JP: Focused attention on brain metastases. Lancet Oncol 10:1024, 2009.
44. Weiss SE, Kelly PJ: Neurocognitive function after WBRT plus SRS or SRS alone. Lancet Oncol 11:220-1, 2010.
45. Sahgal A, Aoyama H, Kocher M, et al: Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: individual patient data meta-analysis. Int J Radiat Oncol Biol Phys 91:710-7, 2015.
46. Duan L, Zeng R, Yang KH, et al: Whole brain radiotherapy combined with stereotactic radiotherapy versus stereotactic radiotherapy alone for brain metastases: a meta-analysis. Asian Pac J Cancer Prev 15:911-5, 2014.
47. Soon YY, Tham IW, Lim KH, et al: Surgery or radiosurgery plus whole brain radiotherapy versus surgery or radiosurgery alone for brain metastases. Cochrane Database Syst Rev 3:CD009454, 2014.
48. Hasan S, Shah AH, Bregy A, et al: The role of whole-brain radiation therapy after stereotactic radiation surgery for brain metastases. Pract Radiat Oncol 4:306-15, 2014.
49. Roberge D, Parney I, Brown PD: Radiosurgery to the postoperative surgical cavity: who needs evidence? Int J Radiat Oncol Biol Phys 83:486-93, 2012.
50. Gans JH, Raper DM, Shah AH, et al: The role of radiosurgery to the tumor bed after resection of brain metastases. Neurosurgery 72:317-25; discussion 325-6, 2013.
51. Pinkham MB, Sanghera P, Wall GK, et al: Neurocognitive Effects Following Cranial Irradiation for Brain Metastases. Clin Oncol (R Coll Radiol) 27:630-9, 2015.
52. Vardy J, Tannock I: Cognitive function after chemotherapy in adults with solid tumours. Crit Rev Oncol Hematol 63:183-202, 2007.
53. van Dam FS, Schagen SB, Muller MJ, et al: Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: high-dose versus standard-dose chemotherapy. J Natl Cancer Inst 90:210-8, 1998.
54. Schagen SB, van Dam FS, Muller MJ, et al: Cognitive deficits after postoperative adjuvant chemotherapy for breast carcinoma. Cancer 85:640-50, 1999.
55. Brezden CB, Phillips KA, Abdolell M, et al: Cognitive function in breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol 18:2695-701, 2000.
56. Ganz PA, Desmond KA, Leedham B, et al: Quality of life in long-term, disease-free survivors of breast cancer: a follow-up study. J Natl Cancer Inst 94:39-49, 2002.
57. Wefel JS, Lenzi R, Theriault RL, et al: The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: results of a prospective, randomized, longitudinal trial. Cancer 100:2292-9, 2004.
58. Meyers CA, Smith JA, Bezjak A, et al: Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trial. J Clin Oncol 22:157-65, 2004.
59. Komaki R, Meyers CA, Shin DM, et al: Evaluation of cognitive function in patients with limited small cell lung cancer prior to and shortly following prophylactic cranial irradiation. Int J Radiat Oncol Biol Phys 33:179-82, 1995.
60. Regine WF, Huhn JL, Patchell RA, et al: Risk of symptomatic brain tumor recurrence and neurologic deficit after radiosurgery alone in patients with newly diagnosed brain metastases: results and implications. Int J Radiat Oncol Biol Phys 52:333-8, 2002.
61. Patchell RA, Tibbs PA, Walsh JW, et al: A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 322:494-500, 1990.
62. Kondziolka D, Patel A, Lunsford LD, et al: Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys 45:427-34, 1999.
63. Mintz AH, Kestle J, Rathbone MP, et al: A randomized trial to assess the efficacy of surgery in addition to radiotherapy in patients with a single cerebral metastasis. Cancer 78:1470-6, 1996.
64. Vecht CJ, Haaxma-Reiche H, Noordijk EM, et al: Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol 33:583-90, 1993.