Corelaţii clinicopatologice în leiomiosarcoamele uterine – prezentare de cazuri şi review de literatură
Clinicopathological correlations in leiomyosarcoma of the uterine corpus – a short series of cases and literature review
Abstract
Uterine sarcomas are a highly aggressive and morphologically heterogeneous group of tumors, with unpredictable clinical course and poor prognosis. According to the latest WHO classification of the tumors of the uterine corpus, uterine sarcomas are grouped into three main histological subtypes: leiomyosarcomas, endometrial stromal sarcomas and other entities. Uterine leiomyosarcoma is a rare type of cancer originating from the smooth muscle tissue of the uterine wall. It is estimated that leiomyosarcoma represents approximately 1% of all uterine malignancies, but it contributes to nearly 70% of all uterine sarcomas and to a significant proportion of uterine cancer deaths. Most uterine leiomyosarcomas occur in women over 40 years old, and their incidence increases rapidly after the age of 50. Due to their scarcity and lack of a pre-invasive stage of the disease, there is no consensus for the screening and early detection of these neoplasms.Keywords
uterine leiomyosarcomaprognostic factorsstageclinicopathologicalIHCRezumat
Sarcoamele uterine sunt un grup de tumori morfologic etergene extrem de agresive, cu o evoluţie clinică imprevizibilă şi cu prognostic slab. Conform celei mai recente clasificări OMS a tumorilor corpului uterin, sarcoamele uterine sunt grupate în trei subtipuri histologice principale: leiomiosarcoame, sarcoame stromale endometriale şi alte entităţi. Leiomiosarcomul uterin este un tip rar de cancer care provine din ţesutul muscular neted uterin. Se estimează că leiomiosarcomul reprezintă aproximativ 1% din toate malignităţile uterine, dar contribuie la aproape 70% din toate sarcoamele uterine şi la o proporţie semnificativă de decese provocate de cancerul uterin. Cele mai multe leiomiosarcoame uterine apar la femeile de peste 40 de ani, iar incidenţa lor creşte rapid după vârsta de 50 de ani. Din cauza rarităţii lor şi a lipsei unor studii efectuate pe loturi populaţionale semnificative statistic, nu există un consens pentru depistarea precoce a acestor neoplasme.Cuvinte Cheie
leiomiosarcom uterinfactori prognosticistadiuclinicopatologicIHCIntroduction
Primitive sarcomas of the uterine corpus are uncommon neoplasms, with an incidence ranging between l% and 3% of all malignancies of the uterine corpus(1). Uterine leiomyosarcomas are softer than leiomyomas in terms of tumor consistency and do not feature a distinct whorled appearance, as leiomyomas do(1,2). Upon microscopic evaluation, areas of hemorrhage and necrosis may be extensive and virtually all tumors express smooth muscle markers like desmin, h-caldesmon, histone deacetulase 8 and smooth muscle actin(2,3).
Although not required for diagnosis, the differences between uterine leiomyosarcomas and uterine leiomyomas include the presence of abnormal p53 expression, a high Ki67 proliferation index and diffuse nuclear staining for p16. In some cases, leiomyosarcomas may show immunoreactivity for CD10 and epithelial markers(2). Other histopathological features including infiltrative borders, coagulative tumor necrosis and nuclear atypia differentiate a uterine leiomyosarcoma from a benign leiomyoma or a smooth muscle tumor of uncertain malignant behavior (STUMP)(2).
Among the risk factors which can be associated with uterine leiomyosarcomas, the most frequent are diabetes and therapy with tamoxifen(4,5). Scientific studies on soft tissue sarcomas discovered an increased risk of developing leiomyosarcoma linked with p53 gene mutations, radiation treatment for childhood cancers and germline mutation in fumarate hydratase(6). Moreover, germline heterozygous loss-of-function mutations of fumarate hydratase predispose to the autosomal dominant syndrome of multiple cutaneous and uterine leiomyomatosis, and in some patients, to renal carcinomas(7).
Clinical features of uterine leiomyosarcoma include abnormal uterine bleeding associated with a palpable uterine mass or pelvic pressure and pain(1,2). Due to the lack of specificity of these symptoms, it is difficult to clinically differentiate between uterine leiomyosarcoma and uterine leiomyomas. The propensity to grow rapidly, either tumor size, or increase of a pre-existing uterine mass are no longer considered risk factors for malignancy(8,9).
Clinical investigations include endometrial biopsy or dilation and curettage, ultrasound, magnetic resonance imaging (MRI) or computed tomography (CT) scan. Endometrial sampling has a sensitivity of 86% and a specificity of 67%(10). Diagnostic accuracy is increased by contrast-enhanced MRI, which has a 94% sensitivity and a 96% specificity(11). Uterine leiomyosarcoma is diagnosed upon histopathological examination of the surgically resected specimen, but frozen section analysis during surgery can lead to the diagnosis(1).
The treatment of uterine leiomyosarcoma is different, depending on the stage of the disease. The gold standard treatment in primary therapy of uterine sarcoma and soft tissue sarcoma is intact “en bloc” surgical resection of the tumor with negative pathologic margins, according to the National Comprehensive Cancer Network(12). In case of uterine-limited disease, a total abdominal hysterectomy with intact uterine removal(3) is recommended. Small laparoscopic for uterine tissue extraction was utilized for enlarged uteri, with benefits such as less blood loss and pain, fewer episodes of venous thromboembolic disease, shorter hospital stays, and faster recovery(13). Adjuvant therapy in the early stages of disease does not improve survival, but it is recommended as palliative treatment.
A multisite, retrospective study of women diagnosed with stage I or stage II uterine leiomyosarcoma treated with radiation therapy, chemotherapy or observation demonstrated no significant difference in recurrence rate based on therapy modality(14). In advanced-stage or recurrent disease, the treatment of uterine leiomyosarcoma depends on the clinical situation(2). In case of surgically resectable disease, radical cytoreductive surgery is recommended, while in the setting of recurrent, oligometastatic disease, surgery should also be strongly taken into consideration(3,12). In case of unresectable or extra-abdominal disease, palliative chemotherapy should be considered(2,3). Currently, in an effort to improve response rate and survival for women with uterine leiomyosarcoma, clinical trials of biologics therapy and immunotherapy are studied.
Case series
The cases presented in this paper were retrieved from the patient files of the Obstetrics and Gynecology Department of the Emergency University Hospital in Bucharest, Romania, using the search terms “uterus” and “leiomyosarcoma”. A total of three cases were identified with these search criteria during a period of 60 days.
Case 1
We present the case of a 68-year-old Caucasian patient who was admitted in the Obstetrics and Gynecology Clinic of the Bucharest University Emergency Hospital for acute urinary retention. No metrorrhagia was documented in the postmenopausal period (menopause installed at the age of 48, considered in the normal range for female population in Romania). She reported no irregular bleeding for the past 20 years and had no family history of any type of cancer.
We also noted general information about lifestyle, heredocolateral history, personal physiological and pathological history, but without any significant data. From her gynecological personal history, we noted a spontaneous vaginal delivery at the age of 20 and a total of three on-demand abortions. She had a Body Mass Index (BMI) of 24, concluding she was a normal-weight patient, non-smoker and non-hypertensive. She was a nurse practitioner for about 35 years. She did not suffer from any other chronic conditions and was not taking any medication at the time of admission.
On examination, she had an enlarged uterus, a distended abdomen and suprapunian tenderness. She required emergency treatment with a urinary catheter, resulting in a urinary output of about 900 ml. The patient failed to void after an attempt of catheter removal, concluding in re-catheterization.
Pelvic transvaginal ultrasound examination revealed an enlarged uterus, measuring 160/100/80 mm, distended by an endometrial inhomogeneous mass counting for about 80 mm. The white blood cell count was 11.30x103/µL, hemoglobin was 13.2 g/dl. The level of CA125 was slightly elevated – 40.7 U/ml (normal range may vary according to age and pre-/postmenopausal status). No other markers for inflammatory status were present and also no other signs of infection – we have not detected the presence of any bacteria in the urine sample, nor in the vaginal or cervical cultures.
An endometrial biopsy was performed, during which approximately 50 g of endometrial material was removed and sent to histopathological examination, revealing a high-grade leiomyosarcoma (G3).
Computed tomography in native and contrast enhanced examination (Figure 1) demonstrated an 88/78/72 mm uterus, containing an endometrial cavity of about 43/38 mm, revealing an inhomogeneous mass (due to air density after the intervention – endometrial biopsy). Myometrial images showed diffuse inhomogeneous enhancement, especially at the posterolateral level. The adnexal mass had no noticeable modification in CT examination. No significant lymphadenopathy and no secondary local bone determination were noted, and also without any evidence of abdominal or lung metastases.
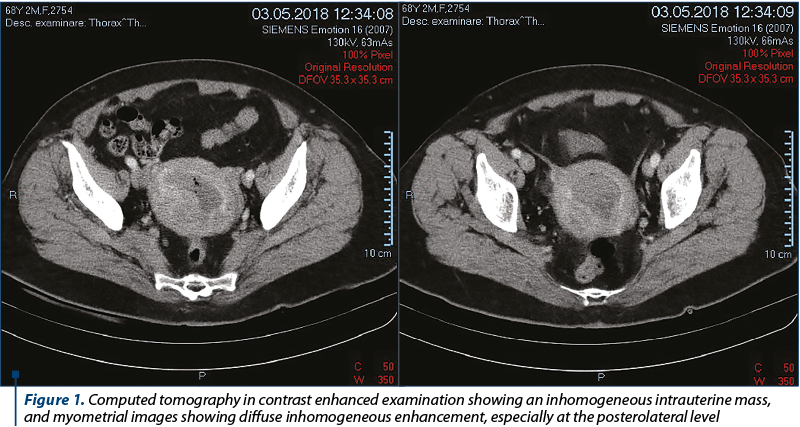
After extensive multidisciplinary counselling, the patient decided to undergo radical hysterectomy and bilateral oophorectomy. The surgically resected specimen was sent to the Department of Pathology in the same clinic, for histopathological examination. Gross inspection revealed a multinodular tumor of the uterine corpus, presenting multiple areas of hemorrhage and necrosis, extending throughout the entire uterine cavity.
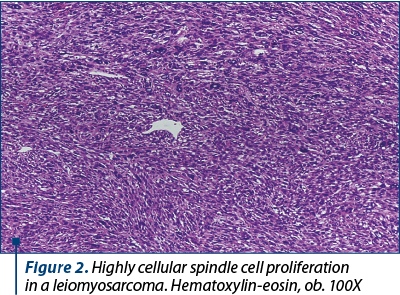
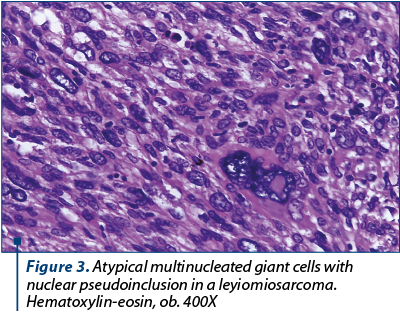
Microscopic examination revealed a high-grade sarcoma composed of elongated smooth muscle cells with moderate to severe nuclear atypia. The tumor cells invaded the deep portion of the myometrium and showed a proclivity for perivascular growth. Also, we observed richly cellular areas with elongated and spindle cells, exhibiting marked pleomorphism with multiple mitotic figures (Figure 4), bizarre giant cells (including few osteoclast-like giant cells) with nuclear hyperchromatism (Figure 2).
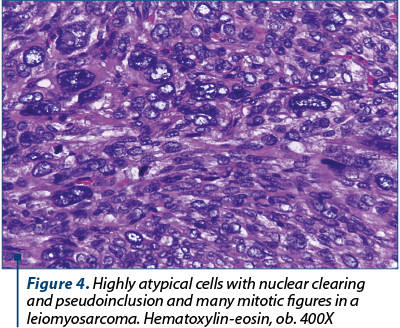
In many areas we noted eosinophilic nuclear pseudoinclusions (Figure 3). Areas of hyaline change, necrosis and myxoid degeneration were also present.
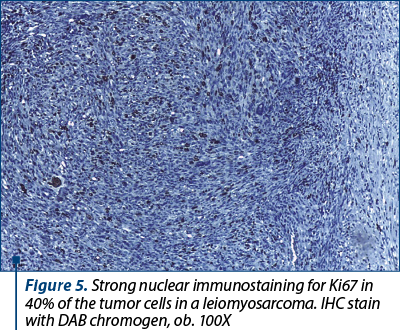
Upon thorough examination of bilateral pelvic lymph nodes (external iliac, obturator and crural), all 32 identified nodes were negative for malignancy, but presented with degenerative changes (lipodystrophy, scarce amyloid deposits, microcalcification) and significant sinus histiocytosis. Extensive immunohistochemical tests were performed and confirmed the diagnosis of leiomyosarcoma (staged pT1b N0 M0). Ancillary tests revealed that the tumor cells showed strong immunoreactivity for smooth muscle actin (SMA), desmin, vimentin, progesterone and estrogen receptors, a high Ki67 proliferative index (Figure 5) and focal overexpression of p53. No CD117 (c-Kit) immunoreaction was noted.
Postoperative recovery was uncomplicated, and the patient is next to follow adjuvant treatment. Considering the absence of secondary dissemination and the early stage of the tumor, the absence of severe cardiovascular disease or renal impairment, the prognosis may be favorable. Still, there is much to see considering the patient age and response to treatment.
Case 2
The second case came from a 60-year-old nulliparous Caucasian woman who was referred to our clinic for metrorrhagia and pelvic pain. General information about lifestyle and family history were unremarkable. In regards to personal medical history, it is important to mention that the patient was hospitalized for the first time in our department 6 months before, for abnormal uterine bleeding.
Further investigations revealed that she suffered from high blood pressure, irritable bowel syndrome, mixed dyslipidemia and a mixed depressive anxiety disorder. She went into menopause at 48 years old and she was also involved in a car crash accident resulting in a right temporal bone fracture at 41 years old.
Recently, she underwent treatment for a genital infection with Streptococcus agalactiae. No weight loss, night sweats and loss of appetite were recorded and there is no family history of malignancy.
Clinical examination revealed an overall healthy-looking woman, with no visible lymphadenopathies. The liver was palpable 3 cm below the costal margin and was not tender. Vaginal examination revealed no untoward clinical features. The clotting profile, blood sugar and renal function were within the normal range. A chest radiograph and a EKG examination showed no modifications.
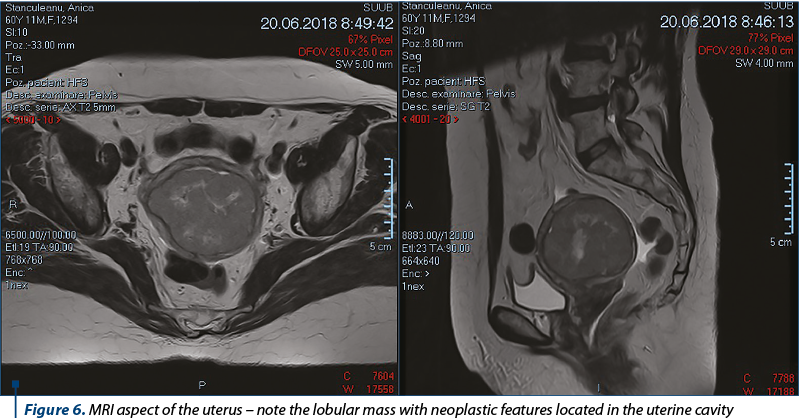
A magnetic resonance imaging (MRI) was undertaken (Figure 6). The MRI of the pelvis showed a globally enlarged uterus (82/72/103 mm), with two intramural fibromatous nodules, and the endometrial cavity being occupied by a lobular mass (66/64/64 mm) with neoplastic features, but with no significant lymphadenopathy and no secondary local bone determination, and also without any evidence of abdominal or lung metastases. The adnexal mass had no noticeable modification in MRI examination.
Upon multidisciplinary evaluation, it was decided that the patient should undergo radical hysterectomy with bilateral adnexectomy. The surgically resected specimen was sent to the Department of Pathology in the same clinic for histopathological examination. Routine examination of paraffin embedded tissue revealed that the tumor was a leiomyosarcoma with a high degree of malignancy, which was focally invasive into the serous layer of the uterus (pT1bNxMx, FIGO stage IB). Osteoclastic-type cells were identified, along with multinuclear pleomorphic cells and intra-tumoral angiogenic proliferation. At the periphery of the lesion we noticed multiple multinucleated bizarre giant cells, reminiscent of a symplastic leiomyoma. This last feature has led us to conclude that the leiomyosarcoma could have originated in a preexisting symplastic leiomyoma. The immunostains confirmed our supposition, as the peripheric areas of the leiomyosarcoma with symplastic features had a low Ki67 proliferative index. The other nodular mass was a benign tumor: a concurrent symplastic leiomyoma (atypical/bizarre leiomyoma), with strong nuclear immunoexpresion of p16 and p53 and very low Ki67 proliferation index. Parcelar HSIL lesions have also been identified in the cervix.
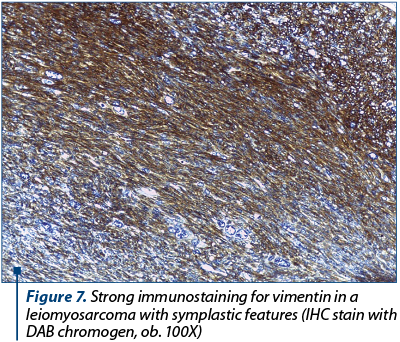
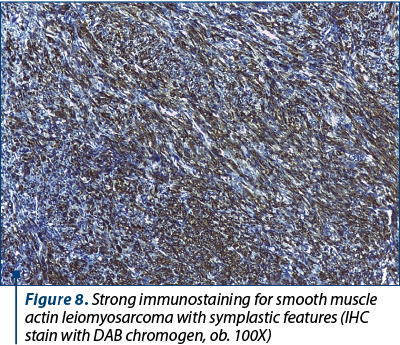
Although in this case immunohistochemistry was not necessary for the diagnosis of leiomyosarcoma, the tumor was tested for several immunohistochemical markers in order to exclude other high-grade uterine sarcomas and to confirm its origin in a preexisting bizarre leiomyoma. The tumor was completely negative for AE1/AE3 pan-cytokeratins, excluding a carcinosarcoma. Approximately 40% of the cancerous cells were positive for Ki-67 and almost all of them were immunoreactive for vimentin (Figure 7), smooth muscle actin (Figure 8) and desmin. The tumoral cells were also diffusely positive for p16 and featured abnormal expression of p53.
Postoperative recovery was uneventful. Due to the lack of secondary dissemination and early stage of the tumor, as well as the absence of severe cardiovascular disease or renal impairment, the prognosis may be favorable.
Case 3
A 51-year-old woman was referred to our Obstetrics and Gynecology Department with complaints of abdominal distension and menorrhagia. From her gynecological personal history, we noted a spontaneous vaginal delivery and two on-demand abortions.
The patient had grade II obesity (IMC=38.06), was a non-smoker, suffering from hypothyroidism and high blood pressure. Laboratory tests included hemoglobin 11.3 g/dl, hematocrit 34.4%, and TSH 4.45 µUI/mL. Vital signs were within normal range.
A low-grade squamous intraepithelial lesion was detected through a routine Pap smear. Upon speculum examination, the cervix appeared normal. Colposcopy also revealed a normal cervix. Ultrasonography scan disclosed a huge solid mass, a 14-cm giant fibromatous nodule with increased vascularization and another fibromatous nodule with a diameter of 3-4 cm that appeared in the cavity. A computed tomography was not performed. A total abdominal hysterectomy with bilateral adnexectomy was performed next, and the surgically resected specimen was sent to the Department of Pathology for histopathological examination.
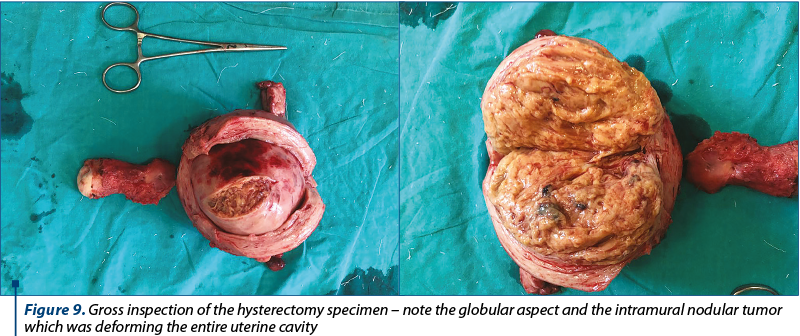
Gross inspection of the hysterectomy specimen revealed a globular uterus of 11/10/9.5 cm, with an intramural nodular tumor which was deforming the entire uterine cavity, featuring an inhomogeneous, whitish-yellow appearance, with hemorrhagic areas and a maximum diameter of 9 cm (Figure 9). The uterine cavity had a length of approximately 7 cm and the endometrial thickness was 0.2 cm.
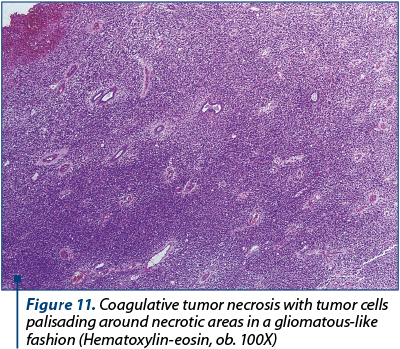
On microscopic examination, the tumor growth showed typical features of a high-grade leiomyosarcoma with multiple hemorrhagic and necrotic areas. Moreover, we noted some peculiar features such as multiple normal endometrial glands entrapped in the sarcomatous proliferation (Figure 10 and Figure 11).

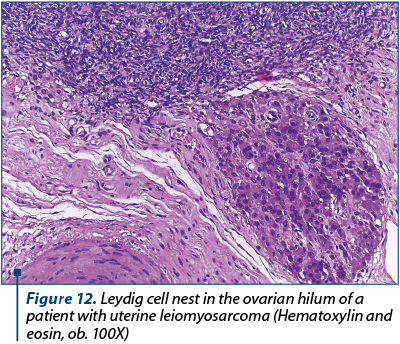
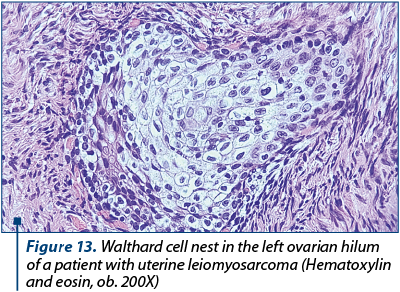
However, this histopathological feature is a common characteristic of adenosarcoma and we performed multiple ancillary tests to exclude the former diagnostic supposition, even though the tumor proliferation was a solid mass which did not exhibit phylloid-like growth pattern and the sarcomatous background was typical of a high-grade sarcoma. The tumor proliferation had an immunostaining pattern characteristic for leiomyosarcoma, with high expression of smooth muscle actin, desmin h-caldesmone, vimentin, progesterone and estrogen receptor. We also noted patchy p53 expression. The sarcomatous growth did not show any expression for CD10, WT1, EMA, CD34 and cytokeratins/Ae1/Ae3, thus excluding an adenosarcoma. The entrapped endometrial glands were highlighted by Ae1/Ae3 cytokeratins and estrogen receptor. Ki67 immunostaining showed almost 90% positive cells, even though on conventional HE examination we observed about 25 mitotic figures per 10 HPF (high power fields). The final diagnosis was high-grade leiomyosarcoma (staged pT1b N0 M0). Other peculiar concomitant microscopic findings were not related with the sarcomatous proliferation: we found multiple nests of Leydig cells (Figure 12) in the right ovary (confirmed by immunohistochemistry). Those cells were strongly positive for inhibin, calretinin and Melan A. The left ovary had multiple Walthard cells nests in the hilum (Figure 13).
Discussion
Uterine sarcomas are very rare neoplasms, accounting for only 3-7% of all uterine malignancies(13-15). They can be broadly classified into leiomyosarcomas, which develop from myometrial smooth muscle, and endometrial stromal tumors, which originate from endometrial stromal cells(16-18). Malignant mixed müllerian tumors or carcinosarcomas feature both epithelial and mesenchymal components and are now thought to be metastatic carcinomas, rather than a subgroup of sarcomas. Leiomyosarcomas are the most common, accounting for approximately 25-36% of all uterine sarcomas, and being well-known as highly aggressive tumors with poor prognosis(19,20). The disease is prevalent in relatively young women, with ages ranging between 40 and 55 years old. Long-term survivors of hereditary retinoblastoma or prior pelvic radiation are considered risk groups. Hormonal conditions such as oral contraceptive use have not been associated with leiomyosarcoma. Most patients present with tumors confined to the uterus. However, even when diagnosed at an early stage, recurrence rate ranges from 53% to 71%(21,22). First recurrences occur in the lungs in 40% of patients and in the pelvis in only 13%. Overall survival rate ranges from 15% to 25%, with a median survival of only 10 months in one study(21,22).
There has been no consensus among various scientific studies regarding the correlation between survival and patient age, tumor stage, size, type of invasive border (pushing vs. infiltrative), presence or absence of necrosis, mitotic rate, degree of nuclear pleomorphism and vascular invasion(23-25). One study found tumor size to be a major prognostic parameter: five out of eight patients with tumors <5 cm in diameter survived, whereas all patients with tumors >5 cm died as a result of the tumor(26). The only other parameters predictive of prognosis were tumor grade and stage(26). Unfortunately, the preoperative diagnosis of uterine leiomyosarcoma has many deficits. Although the diagnosis may be suspected following imagistic methods, the histopathological examination is essential in establishing the diagnosis(27-29). Histopathological examination through endometrial sampling features a low sensitivity of approximately 30% and is unreliable for patients suspected of leiomyosarcoma, as it cannot offer a precise result, unless the patient has a late-stage disease and the tumor has already reached the surface of the endometrial cavity. Similarly, examination of intraoperative frozen sections performed for suspicious fibroids are often inaccurate(30). The histopathological diagnosis of uterine leiomyosarcoma is generally straightforward since most tumors exhibit the microscopic criteria such as hypocellularity, severe cito-nuclear atypia and high mitotic index usually exceeding 15 mitoses per 10 high-power fields(26). In addition, one or more clinical or morphological features, such as advanced age at the diagnosis, extrauterine extension, tumor size over 10 cm in diameter, infiltrating borders, tumor coagulative necrosis and atypical mitotic figures, are also present(26). Moreover, the minimal pathological criteria for the correct diagnosis of a leiomyosarcoma can be problematic, and in such cases the differential diagnosis must consider a constellation of smooth muscle proliferations that exhibit unusual features(26). Those specific subtypes of leiomyoma that can mimic malignancy are: mitotically active leiomyoma, cellular leiomyoma, haemorrhagic or apoplectic leiomyoma, symplatic (bizarre) leiomyoma, leiomyoma with myxoid or epithelioid features, leiomyoma with a massive lymphoid background and, finally, smooth muscle tumors of uncertain malignant potential (STUMP)(2,26). Sometimes, the histopathological aspect of highly aggressive leiomyosarcomas may resemble that of serous endometrial carcinomas or high-grade serous ovarian and fallopian tube carcinomas(26).
Uterine leiomyomas are not broadlly thought to evolve into malignant tumors such as leiomyosarcomas, but leiomyosarcomas frequently coexist or are concomitant with other leiomyomas, and about 0.5% of women who have hysterectomies for uterine fibroids are found to also have leiomyosarcomas(31). More and more studies suggest that indeed some leiomyosarcomas may arise from preexisting benign lesions such as bizarre leiomyomas. A study performed by Mittal et al. brought strong molecular and immunohistochemical evidence that some leiomyosarcomas can arise from associated leiomyoma or from leiomyoma with symplastic-like areas(32). One of our cases is similar with one included in this study. Moreover, the fact that our patient had also a synchronous symplastic/bizarre leiomyoma is a strong evidence that those malignancies may arise from pre-existing benign lesion with unusual features.
Epithelioid and myxoid leiomyosarcomas are two rare histologic variants which lack the severe nuclear atypia and high mitotic rate of the usual spindle-shaped leiomyosarcomas.
Epithelioid leiomyosarcomas are constituted predominantly or entirely of round or polygonal cells with eosinophilic or clear cytoplasm which grow diffusely in nests, cords or feature a plexiform pattern. Mitotic rate is usually <3 mitotic figures per 10 HPF. The majority of tumors invade the adjacent myometrium, but lymphovascular invasion is usually rare.
Myxoid leiomyosarcomas are rare smooth muscle tumors with abundant myxoid stroma. Upon gross examination, the tumors are usually large, gelatinous and well circumscribed. Microscopically, they differ from conventional leiomyosarcomas and feature a hypocellular and myxoid appearance. The typically low mitotic count in these tumors is largely due to the separation of cells by the abundant myxoid stroma; however, myxoid leiomyosarcomas are almost always malignant. Leiomyosarcomas usually present as solitary lesions and only exceptionally transform from a leiomyoma or, as in one of our cases, from a smooth muscle tumor of uncertain malignant potential. However, it is not as unusual to identify two neoplasms in the same specimen. Leiomyosarcomas and leiomyomas have completely different molecular pathways. Although, to date, the exact pathogenesis of leiomyosarcoma is not completely understood, current scientific results demonstrated high genetic instability, with multiple abnormalities in p16 and p53.
Although infrequent, leiomyosarcomas are highly malignant neoplasms with increased recurrence rates, even in the early stages of the disease. If diagnosed preoperatively, patients should undergo total hysterectomy with bilateral salpingo-oophorectomy. Lymphadenectomy should be undertaken only in patients with suspicious nodes or extrauterine disease.
Conclusions
We strongly believe that further development of advanced imaging techniques to preoperatively differentiate between benign leiomyomas and leiomyosarcomas will provide significant prognostic data for the patient. Moreover, further molecular biology research of the etiopathogenetic pathways and receptor status may deliver fundamental insights on possible targeted therapies to improve the overall outcome of patients diagnosed with leiomyosarcoma.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
- Kurman RJ, Carcangiu ML, Herrington S, Young RH. Who Classification of Tumors of female Reproductive Organs, 4th Ed. Lyon; IACR, 2014; 135-9.
- Forough F, Abolfazl MK, Kambiz S, Mania K, Mehrangiz Gi. Uterine Leiomyosarcoma: A case Report. Internat J Wom Health Reprod Scienc. 2018; 6(2):223-5.
- Ricci S, Stone RL, Fader An. Uterine leiomyosarcoma: Epidemiology, contemporary treatment strategies and the impact of uterine morcellation. Gynec Oncol. 2017, 145(1):208-16.
- Felix AS, Cook LS, Gaudet MM. The etiology of uterine sarcomas: a pooled analysis of the epidemiology of endometrial cancer consortium. Br. J. Cancer. 2013; 108(1):727-34.
- Lavie O, Barnett-Griness O, Narod SA. The risk of developing uterine sarcoma after tamoxifen use. Int. J. Gynecol. Cancer. 2008; 18(2):352-6.
- Abeler VM, Røyne O, Thoresen S. Uterine sarcomas in Norway. A histopathological and prognostic survey of a total population from 1970 to 2000 including 419 patients. Histopathology. 2009; 54 (3):355-64.
- Alam NA, Olpin S, Leigh IM. Fumarate hydratase mutations and predisposition to cutaneous leiomyomas, uterine leiomyomas and renal cancer. Br J Dermatol. 2005; 153(1):11-7.
- Dossi R, Frapolli S, Di Giandomenico S. Antiangiogenic activity of trabectedin in myxoid liposarcoma: involvement of host TIMP-1 and TIMP-2 and tumor thrombospondin-1. Int. J. Cancer. 2015; 136(2):721-9.
- Monk BJ, Blessing JA, Street DG, Muller CY, Burke JJ, Hensley ML. A phase II evaluation of trabectedin in the treatment of advanced, persistent or recurrent uterine leiomyosarcoma: a gynecologic oncology group study. Gynecol. Oncol. 2012; 124:48-52.
- Bansal N, Herzog TJ, Burke W, Cohen CJ, Wright JD. The utility of preoperative endometrial sampling for the detection of uterine sarcomas. Gynecol. Oncol. 2008; 110(1):43-7.
- Lin G, Yang LY, Huang YT. Comparison of the diagnostic accuracy of contrast-enhanced MRI and diffusion-weighted MRI in the differentiation between uterine leiomyosarcoma/smooth muscle tumor with uncertain malignant potential and benign leiomyoma. J. Magn. Reson. Imaging. 2016, 43(2):333-42.
- Koh Wh, Greer BR, Abu-Rustum NR, Apte SM, Campos SM, Cho KR. Uterine sarcoma, version 1.2016: featured updates to the NCCN guidelines. J Natl Compr Cancer Netw. 2015, 13(11):1321-31.
- AAGL position statement: route of hysterectomy to treat benign uterine disease, J. Minim. Invasive Gynecol. 2011; 18(2):1-3
- Ricci S, Giuntoli RL, Eisenhauer E, .Lopez MA, Krill L, Tanner EJ. Does adjuvant chemotherapy improve survival for women with early-stage uterine leiomyosarcoma? Gynecol Oncol. 2013; 131(1):629-33
- Zaloudek C, Norris HJ. Mesenchymal tumors of the uterus. In: Blaustein’s Pathology of the Female Genital Tract. 1994; 29:487-528.
- Foley OW, Rauh Hain A, Clemmer J, Clark RM, Hall T, Diver EJ, Schorge JO, del Carmen MG. Trends in the treatment of uterine leiomyosarcoma in the medicare population. Int J Gynecol Cancer. 2015; 25(3):453-8.
- Livi L, Paiar F, Shah N, Blake P, Villanucci A, Amunni G, et al. Uterine sarcoma: twenty-seven years of experience. Int J Radiat Oncol Biol Phys. 2003; 57:1366–73.
- Acharya S, Hensley ML, Montag AC, Fleming GF. Rare uterine cancers. Lancet Oncology. 2005; 6:961–71.
- Norris HJ, Zaloudek CJ. Mesenchymal tumors of the uterus. In: Blaustein A, edit. Pathology of the Female Genital Tract. 2nd ed. NY Springer-Verlag Books; 1982:352–92.
- Echt G, Jepson J, Steel J, Langholz B, Luxton G, HernandezW, et al. Treatment of uterine sarcomas. Cancer. 1990; 66:35–9.
- Zivanovic O, Jacks LM, Iasonos A, et al. A nomogram to predict postresection 5-year overall survival for patients with uterine leiomyosarcoma. Cancer. 2012; 118:660-9.
- Major FJ, Blessing JA, Silverberg SG, et al. Prognostic factors in early-stage uterine sarcoma: a gynecologic oncology group study. Cancer. 1993; 71:1702-9.
- Mayerhofer K, Obermair A, Windbichler G, et al. Leiomyosarcoma of the uterus: a clinicopathologic multicentre study of 71 cases. Gynecol Oncol. 1999; 74:196-201.
- D’Angelo E, Spagnoli LG, Prat J. Comparative clinicopathologic and immunohistochemical analysis of uterine sarcomas diagnosed using the World Health Organization classification system. Hum Pathol. 2009; 40:1571-85.
- Wang WL, Soslow R, Hensley M, et al. Histopathologic prognostic factors in stage I leiomyosarcoma of the uterus: a detailed analysis of 27 cases. Am J Surg Pathol. 2011; 35:522-9.
- Giuntoli 2nd RL, Gostout BS, DiMarco CS et al. Diagnostic criteria for uterine smooth muscle tumors: leiomyoma variants associated with malignant behaviour. J Reprod Med. 2007; 52:1001-10.
- Toro JR, Travis LB, Wu HJ, Zhu K, Fletcher CDM, Devesa SS. Incidence patters of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978–2001: an analysis of 26,758 cases. Int J Cancer. 2006; 119:2922-35.
- Kelley TW, Borden EC, Goldblum JR. Estrogen and progesterone receptor expression in uterine and extrauterine leiomyosarcomas: an immunohistochemical study. Appl. Immunohistochem Mol Morphol. 2004. 12:338-41.
- Major FJ, Blessing JA, Silverberg SG, Morrow CP, Creasman WT, Currie JL, Yordan E, Brady MF Prognostic factors in early-stage uterine sarcoma. A Gynecologic Oncology Group study. Cancer. 1993; 71 (4 Suppl):1702.
- Leibsohn S, d’Ablaing G, Mishell DR Jr, Schlaerth JB. Leiomyosarcoma in a series of hysterectomies performed for presumed uterine leiomyomas. Am J Obstet Gynecol. 1990; 162:968–74; discussion 974–6.
- Harry VN, Narayansingh GV, Parkin DE. Uterine leiomyosarcomas: A review of the diagnostic and therapeutic pitfalls. Obstet Gynaecol. 2007; 9:88–94.
- Mittal KR, Chen F, Wei JJ, Rijhvani K, Kurvathi R, Streck D, Dermody J, Toruner GA. Molecular and immunohistochemical evidence for the origin of uterine leiomyosarcomas from associated leiomyoma and symplastic leiomyoma-like areas. Mod Pathol. 2009; 22(10):1303-11.

