In patients with acute leukemia, neurological emergencies are redundant complications that can aggravate the course of the underlying disease or cause death if they are not diagnosed on time. The article proposes to discuss and illustrate the neurological emergencies that may be encountered in patients with acute leukemia. Computed tomography (CT) and magnetic resonance imaging (MRI) are the imaging modalities of choice used for the positive diagnosis of these complications, CT examination being particularly indicated in patients suspected with intracranial hemorrhage, as well as in agitated and uncooperative patients, and MRI being performed either in addition to the CT evaluation, or in cases with suspicion of infectious-inflammatory lesions or cerebral venous thrombosis.
Urgenţele neurologice în leucemiile acute: ce este important să ştim?
Neurological emergencies in acute leukemia: what is important to know?
First published: 16 mai 2019
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/OnHe.47.2.2019.2323
Abstract
Rezumat
La pacienţii cu leucemie acută, urgenţele neurologice reprezintă complicaţii redutabile care pot agrava cursul bolii de bază sau pot produce decesul dacă nu sunt diagnosticate la timp. Articolul îşi propune discutarea şi ilustrarea urgenţelor neurologice care pot fi întâlnite la pacienţii cu leucemii acute. Evaluarea computer-tomografică (CT) şi prin rezonanţă magnetică (IRM) reprezintă modalităţile imagistice de elecţie utilizate în diagnosticul pozitiv al acestor complicaţii, examinarea CT fiind indicată în special pentru pacienţii la care se suspicionează hemoragie intracraniană, precum şi la pacienţii agitaţi, necooperanţi, iar IRM-ul este realizat fie în completarea evaluării CT, fie în cazul suspectării de leziuni de tip infecţios-inflamator sau în caz de tromboză venoasă cerebrală.
Purpose
To review and illustrate computed tomography and magnetic imaging features encountered in patients with acute leukemia (AL) which develop acute brain complications.
Introduction
Leukemias are a heterogeneous group of hematologic malignancies resulting from a neoplastic proliferation of hematopoietic cells at an undifferentiated or partially differentiated stage of maturation(1). Patients with acute leukemia (AL) have an increased risk of developing a disease of the central nervous system (CNS). The AL are classified as myeloid or lymphoid on the lineage of the blast cells(1-3). The overall incidence of acute leukemia is approximately 4/100,000 people per year, with 70% of these cases being acute myeloid leukemia (AML) which occur in adults. Acute lymphoblastic leukemia (ALL) is predominantly a disease of children, 75% of cases usually under 6 years old(3). The possible etiologic factors associated with leukemia include viruses, ionizing radiation, cytotoxic chemotherapy and benzene(1). Approximately 25-50% of patients with AL will suffer a central nervous system (CNS) complication at some point in the course of the disease(3-6). In AL patients, with acute and progressive neurologic signs and symptoms, the differential diagnosis includes: direct spread to the craniocerebral region of the leukemic cells; cerebrovascular complications, infectious, and treatment-related complications(4-6). More than one type of lesion may coexist.
Imaging. Knowledge of the CNS leukemic acute complications spectrum and their imaging findings (computed tomography and magnetic resonance imaging) is necessary to achieve a correct diagnosis in the leukemic patients who present neurologic signs(4-9).
Computed tomography (CT). Nonenhanced and/or enhanced brain CT (after i.v. injection of 1.5 ml/kg non-ionic iodinated contrast material) protocol must be done according to the age of the patient and according to the unenhanced CT aspects.
Magnetic resonance imaging (MRI). Brain MRI protocols consist of nonenhanced T1, T2, Flair, T2 EG/SWI and diffusion weighted sequences and, in selected cases, 3D T1 precontrast and after i.v. injection of paramagnetic Gd contrast material (0.1 ml/kg).
Neurological emergencies in acute leukemia. The neurological emergencies in AL include cerebrovascular emergencies and infections secondary to immunodeficiency, in the differential diagnosis entering intracranial tumoral infiltrations and complications due to treatment (radiotherapy, chemotherapy and after bone marrow transplantation). Cerebrovascular events are among the most severe CNS complications in patients with AL and contribute to the morbidity and mortality of the disease(4-6). Every coagulation factor has been reported to be abnormally elevated or decreased in patients with AL, and multiple coagulopathies are common in the same patient(4-9). The mechanisms involved in the alteration of coagulation factors include interference with production, accelerated breakdown, and the effect of chemotherapeutic agents, that can result in a hypercoagulable state or, alternatively, in a bleeding diathesis. AL patients may develop disseminated intravascular coagulation (CID), manifested by hypofibrinogenemia, thrombocytopenia, and bleeding from multiple sites, including, sometimes, the CNS(5). Cerebrovascular complications in AL patients include: hemorrhage (intraaxial or extraaxial), dural sinus thrombosis, and cerebral infarctions(4-13).
Intracranial hemorrhage. Intracerebral hemorrhage (ICH) – Figure 1. Patients with ICH may present a sudden onset of headache or neurologic deterioration and seizures.
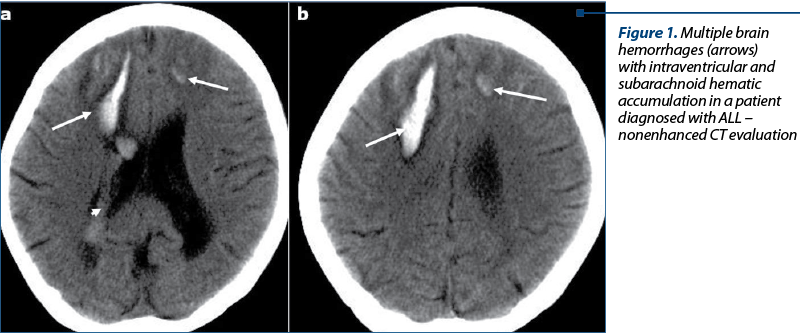
Patients with acute promyelocytic leukemia are at risk for massive intracerebral hemorrhage, which represents the cause of death in more than 60% of patients(6). In AL patients who have disseminated intravascular coagulation, a pattern of multiple small hemorrhages with small collars of edema may be seen in the subcortical white matter, difficult to differentiate only on NECT images from leukemic nodular lesions.
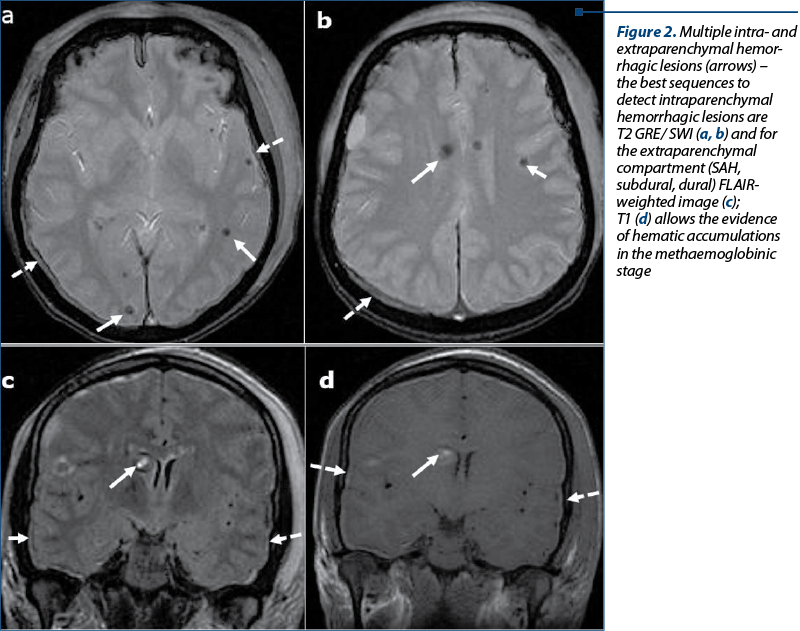
Extraaxial hemorrhage – Figure 2. Extraaxial hemorrhage is far less common, and may be dural, subdural or subarachnoid(6-13).
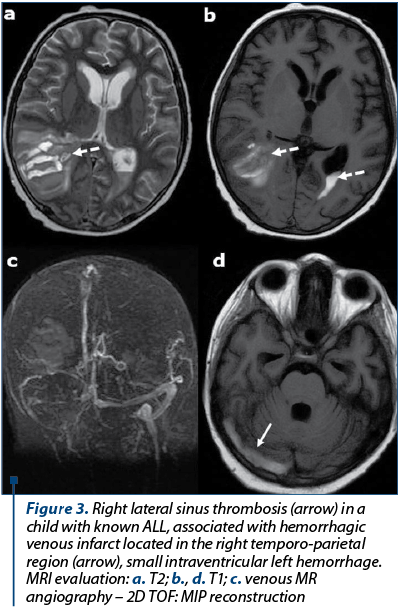
Dural sinus thrombosis. Patients with AL are at increased risk for dural sinus thrombosis (DST) and cortical venous thrombosis. The occlusion of DST in AL patients include CNS infiltration, leukostasis, and chemotherapeutic agents, particularly L-asparaginase, which is known to produce numerous hemostatic defects, including hypofibrinogenemia, prolonged clotting time and multiple clotting factor deficiencies. The reason for thromboses in patients receiving L-asparaginase is poorly understood(9-12). Coagulopathies have also been reported with vincristine and prednisone therapy in leukemic patients. Signs and symptoms of DST such as headache, papilledema, and lethargy can mimic CNS leukemic infiltration, and imaging may be required for differentiation(5,6). At CT, signs of DST are represented by abnormal hyperdensity of a sinus on precontrast scans, abnormal central hypodensity of a sinus on postcontrast CT evaluation (empty delta sign), or excessive meningeal collaterals. MRI findings of the dural sinus thrombosis are: abnormal signal intensity or lack of flow void on precontrast images and empty delta sign (enhancing dura surrounds non enhancing thrombus) on postcontrast MR images(13). Brain hemorrhage or venous infarct may complicate an intracranial dural sinus thrombosis – Figure 3.
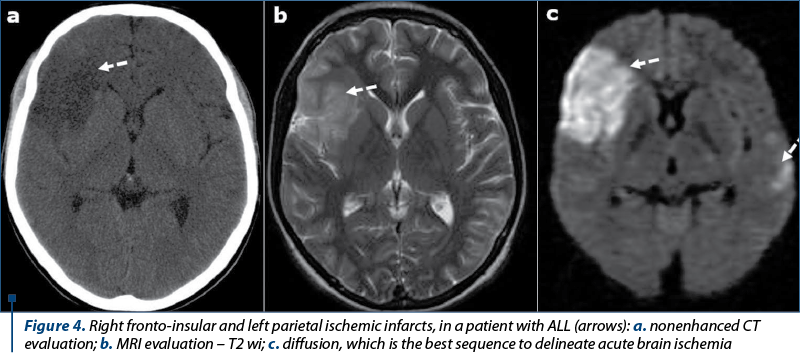
Cerebral infarction. Patients with AL are at increased risk for cerebral infarction (Figure 4), although cerebral infarction is far less common than hemorrhagic events(5-9). The causes are represented by: nonbacterial thrombotic endocarditis, intravascular coagulation, SDT, tumor emboli, septic emboli, miscellaneous (vasculitis, artery compression by a tumor), and L-asparaginase therapy(10-13).
Infections. Patients with AL are susceptible to infections, considering a number of reasons, such as abnormal or decreased granulocytes, mucosal damage (mucositis related to chemotherapy, rendering mucosal surfaces vulnerable to microbial invasion), steroid therapy, and diminished mucociliary clearance(14-18). Sinus infection. Sinusitis are common in AL patients(16). Typical organisms affecting the paranasal sinuses and other mucosal regions are the Gram-negative bacilli such as Escherichia coli, Pseudomonas aeruginosa and Klebsiella pneumoniae, and the Gram-positive Staphylococcus aureus(16). Aspergillus sinusitis can produce various symptoms and may be accompanied by a foul-smelling discharge(17,18). In AL or immunocompromised patients, this infection may be aggressive, resulting in bone destruction and extension into adjacent spaces or intracranial extension.
Intracranial infection can occur by a number of routes in AL patients. The most important routes are represented by direct intracranial spread in aggressive sino-nasal infections and hematogenous intracranial spread(5-8). Infections caused by Aspergillus or the Zygomycetes fungal groups such as Mucor, Candida and Aspergillus organisms are associated with bone destruction, vascular invasion and rapid intracranial spread, causing meningitis, brain abscess, ischemic or hemorrhagic infarction. The progression from sinusitis to intracranial spread and death can occur in just a few days in AL patients(16-18). Hematogenous intracranial spread can occur with various organisms, including fungal species such as Aspergillus, Cryptococcus and Candida, bacteria such as Listeria monocytogenes, and viral species such as varicella zoster(17). Endocarditis from systemic fungemia can result in septic emboli and subsequent brain abscess(18). Abscesses typically appear on CT scans as areas of low density, single or multiple, with peripheral ring-like enhancement. At MR imaging, abscesses are typically hypointense on T1-weighted images and hyperintense on T2-weighted images, sometimes with a ring of signal hypointensity on the T2-weighted images that enhance after paramagnetic Gd contrast material i.v. injection associated with edema. Three imaging patterns have been described in neutropenic patients with cerebral invasive aspergillosis(13,17): a) cortical-subcortical hypoattenuating areas on CT scans or hyperintense areas on T2-weighted images; b) multiple ring-enhancing lesions (Figure 5); c) dural enhancement adjacent to sinonasal disease.
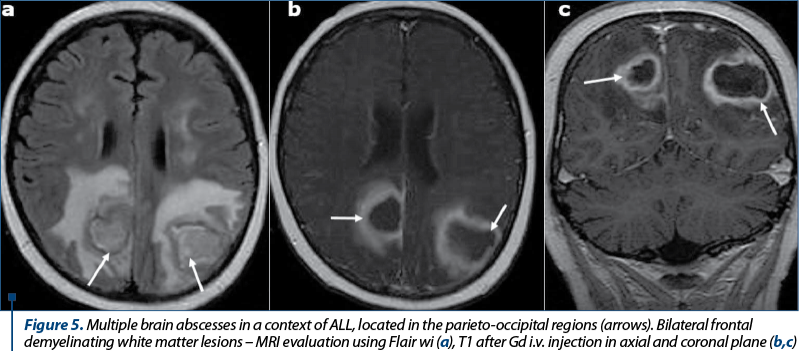
Candidiasis can also be seen in immunocompromised patients(7). It appears as numerous ring-enhancing microabscesses less than 3 mm in diameter at the gray-white matter junction, basal nuclei and cerebellum. Candida affects more frequently vascular structures, causing vasculitis, intraparenchymal hemorrhage, mycotic aneurysms, and thrombosis of small vessels with secondary infarction(17).
Conclusions
In AL patients with acute neurological signs, imaging plays an essential role in establishing the positive diagnosis, being essential for a correct therapy and for an increased overall survival.
CT evaluation represents the first step, indicated for patients suspected with intracranial hemorrhage, as well as for agitated and uncooperative patients. MRI is performed either in addition to the CT evaluation in order to characterize inconclusive brain lesions, or in cases with suspicion of infectious-inflammatory lesions or with cerebral venous thrombosis. A multidisciplinary approach is essential in customized management of patients with AL and acute neurological complications.
Conflict of interests: The author declares no conflict of interests.
Bibliografie
- Laosai J, Chamnongthai K. Classification of acute leukemia using medical-knowledge-based morphology and CD marker. Biomedical Signal Processing and Control. 2018; 44, 127–137.
- Mwirigi A, Dillon R, Ra K. Acute leukaemia. Medicine. 2017; 45:5, published by Elsevier Ltd., 280-286.
- Linet MS, Devesa S. Descriptive epidemiology of the leukemias. In: Henderson ES, Lister TA (eds). Leukemia, 5th ed. Philadelphia: Saunders. 1990; 207-224.
- Vincent PC. Pathophysiology. In: Henderson HS. Lister TA (eds). Leukemia, 5th ed. Philadelphia: Saunders. 1990; 19-34.
- Chen CY, Zimmerman RA, Faro S, et al. Childhood Leukemia: Central Nervous System Abnormalities during and after Treatment. AJNR. 1996; 17:295–310.
- Ginsberg E, Leeds NE. Neuroradiology of Leukemia. AJR. 1995; 165:525-534.
- Vasquez E, Lucaya J, et al. Neuroimaging in Pediatric Leukemia and Lymphoma: Differential Diagnosis. RadioGraphics. 2002; 22:1411–1428.
- Levine GA, Winkelstein A, Shadduck RK. CNS involvement as the initial manifestation of acute leukemia. Cancer. 1973; 31:959-962.
- Nabavizadeh SA, Stein J, Mohan S. Neuroimaging in Leukemia. Hematol Oncol Clin North Am. 2016 Aug; 30 (4):823-42.
- Gugliotta L, Mazzucconi MG, Leone G, et al. Incidence of thrombotic complications in adult patients with acute lymphoblastic leukaemia receiving L-asparaginase during induction therapy: a retrospective study. Eur J Haematol. 1992; 49:63–66.
- Kingma A, Tamminga RY, Kamps WA, Le Coultre R, Saan RJ. Cerebrovascular complications of L-asparaginase therapy in children with leukemia: aphasia and other neuropsychological deficits. Pediatr Hematol Oncol. 1993; 10:303–309.
- Feinberg WM, Swenson MR. Cerebrovascular complications of L-asparaginase therapy. Neurology. 1988; 38:127–133.
- Lupescu IG, Lupescu IC, Gherguş AE, et al. Cranio-cerebral abnormalities in acute leukemia: current and particular CT and MRI aspects. ECR. 2012; doi.org/10.1594/ecr2012 /C-2548.
- Schimpff SC. Infection in the leukemia patient: diagnosis, therapy and prevention. In: ES Henderson, TA Lister (eds.). Leukemia, 5th ed. Philadelphia: Saunders. 1990; 687-709.
- Som PM. Sinonasal cavity. In: Som PM, Bergeron RT (eds.). Head and neck imaging, 2nd ed. St. Louis: Mosby. 1991; 51-291.
- Zinreich SJ, Kennedy DW, Malat J, et al. Fungal sinusitis: diagnosis with CT and MR imaging. Radiology. 1988; 169:439-444.
- Ashdown BC, Tien RD, Felsberg GJ. Aspergillosis of the brain and paranasal sinuses in immunocompromised patients: CT and MR imaging findings. AJR. 1994; 162:155–159.
- Orlowski HLP, McWilliams S, Mellnick VM. Imaging Spectrum of Invasive Fungal and Fungal-like Infections. RadioGraphics. 2017; 34 (4): 1119-1133.
Articole din ediţiile anterioare
Agregometria în diagnosticul sindroamelor hemoragipare
Agregarea trombocitelor este una dintre primele etape în coagularea sanguină şi reprezintă fenomenul de asociere intertrombocitară sub acţiun...
Ghid de diagnostic şi tratament în cancerul sânului
Ghidul se adresează medicilor oncologi care, în concepţia Societăţii Europene de Oncologie Medicală (ESMO), sunt „specialiştii în cancer” care urmă...
Carcinom mucinos cutanat - prezentare de caz
Carcinomul mucinos cutanat primar este o tumoră malignă foarte rară ce afectează cel mai frecvent zona periorbitală, cu originea din zona profundă ...
Carcinomul pulmonar scuamos
Cancerul pulmonar fără celule mici scuamos este unul dintre cancerele care nu are încă un avantaj cert în cadrul terapiei țintite. Deși rezultatele...