Metformin is one of the most used antidiabetic drugs, with extensive experience, multiple benefits and low costs. The issue of the benefits and risks of metformin administration during pregnancy is raised more and more frequently. Although more research is needed, we wanted to review the existing research data for both gestational diabetes and pre-existing diabetes.
Diabetul gestaţional şi diabetul preexistent sarcinii – abordare din perspectiva metforminului
Gestational diabetes and pre-existing diabetes – an approach from the metformin perspective
First published: 12 decembrie 2019
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/Gine.26.4.2019.2709
Abstract
Rezumat
În diabetologie, metforminul este utilizat frecvent, existând o vastă experienţă în utilizarea sa, cu multiple beneficii şi costuri reduse. Din ce în ce mai des, se ridică problema beneficiilor şi a riscurilor administrării metforminului în timpul sarcinii. Deşi mai multe cercetări sunt necesare, am dorit să revizuim cercetările existente atât din punctul de vedere al diabetului gestaţional, cât şi al diabetului preexistent sarcinii.
Introduction
Metformin is one of the most used antidiabetic drugs, with extensive experience, multiple benefits and low costs. Both pre-existing diabetes and gestational diabetes are on the rise. Although initially metformin was not used in pregnancy due to the lack of studies, issues related to safety and the degree of passage of the placenta, the actual perspective is different.
Hyperglycemia, even mild, during pregnancy is associated with an increase in maternal, fetal and neonatal adverse effects, with a change in lifestyle, self-monitoring of blood glucose and pharmacological intervention for glycemic control(1,2). Usually, insulin is the therapeutic option in gestational diabetes, being safe for the mother and the fetus. However, metformin is an effective, easier to administer and less expensive option, which is increasingly used by clinicians for gestational diabetes.
One of the first mentions related to the administration of metformin in gestational diabetes was in 1975 at the Aberdeen International Symposium on Carbohydrate Metabolism in Pregnancy and Newborn(3). In 1980, Coetzee et al. conducted observational studies regarding the safety and efficacy of metformin administration in pre-existing diabetes and gestational diabetes in women from South Africa(4,5).
Subsequently, several studies have shown the efficacy and lack of side effects when administering metformin during pregnancy. These studies have shown that the use of metformin does not increase the risk of miscarriage; metformin crosses the placenta, which can expose the fetus. The long-term effects on fetal programming and the offsprings of children exposed to intrauterine metformin are not completely elucidated(6,7).
The action of metformin in the context of pregnancy
Metformin improves insulin sensitivity, reduces hepatic gluconeogenesis and increases peripheral glucose uptake, with a minimal risk of hypoglycemia, and can reduce the body weight by an average of 5.8%(8). In the context of pregnancy, it is important to note that the placenta expresses many isoforms of organic cationic transporters (OTC) and therefore metformin readily crosses the placenta(9). Fetal metformin concentrations can reach at least 50% of the maternal concentration(10).
Placental transport to the developing fetus raises the question of potential adverse effects on fetal development. At present, it is not known whether the human embryo expresses OTC; pre-implanted human embryos have a low mitochondrial content and may therefore be unresponsive to metformin(11). Metformin stimulates the 5’-adenosine monophosphate-activated protein kinase (AMPK) activity in embryonic stem cell cultures in mice in vitro, but the significance of this finding is unclear, given the lack of effect on mouse embryos in vivo(12,13).
The renal clearance of metformin is significantly increased in the second half of pregnancy, consistent with increased renal plasma flow and glomerular filtration during pregnancy(14). For a proper glycemic control, patients with gestational diabetes may require high doses of metformin (500-2500 mg/day). At doses above 2500 mg/day, maternal, fetal or newborn safety is unknown.
Type 2 diabetes pre-existing pregnancy and metformin
The prevalence of type 2 diabetes is increasing, the age of onset of type 2 diabetes is decreasing, and the age of pregnant women is increasing, all of which lead to an increasing prevalence of pregnant women with type 2 diabetes(15). These women have an increased risk of obesity, pregnancy-induced hypertension, preeclampsia and caesarean section(16).
Because metformin is a first-line therapy for type 2 diabetes, many future pregnant women with pre-existing diabetes are on metformin treatment, raising the issue of metformin administration during pregnancy. On the other hand, given the insulin resistance of pregnancy, it is likely that most women with type 2 diabetes prior to pregnancy require insulin treatment during pregnancy, to maintain glycemic control.
Even though insulin is needed, metformin may contribute to improved maternal glycemic control, better maternal weight control, and reduced insulin dose. In women receiving metformin prior to pregnancy, the discontinuation of it may lead to glycemic fluctuations and increased insulin dose, especially in the second half of pregnancy, when an additional physiological decrease in insulin sensitivity occurs.
Hellmuth et al. conducted a retrospective study between 1966 and 1984, including 50 women (19 with pre-existing type 2 diabetes), and have noted an increase in preeclampsia and perinatal mortality with metformin compared to sulphonylurea or insulin treatment(17).
Levitt et al. followed women with pre-existing type 2 diabetes under treatment with insulin or oral antidiabetic agents before and during pregnancy. There was a very high rate of perinatal mortality (125 to 1000 births) in the group treated predominantly with metformin and glibenclamide; women who switched to insulin from oral medication had a perinatal mortality rate of 28 to 1000 births and in women who switched from diet to insulin the rate was 33 to 1000 births(18).
Hughes and Rowan were unable to demonstrate any difference in maternal and fetal adverse outcomes in women taking metformin compared to those taking insulin(19).
The National Institute for Health and Care Excellence (NICE) recommends that women with pre-existing type 2 diabetes receive metformin as an adjuvant or alternative to insulin during the preconception and during pregnancy, when the likely benefits of improved glucose control outweigh the potential for adverse effects(20).
Gestational diabetes and metformin
Gestational diabetes represents the diabetes that occurs during pregnancy, its severity differing from person to person. The prevalence of gestational diabetes is increasing worldwide, as the age at which women become pregnant increases and the prevalence of obesity increases(21).
Rowan et al. managed to change the perspective on the use of metformin in pregnant women; they studied 751 women with gestational diabetes, who were randomized to either metformin or insulin. Most women with metformin required supplemental insulin (46%), but at considerably lower doses than women receiving insulin alone. Gastrointestinal adverse reactions resulted in the discontinuation of metformin in 1.9% of women and in dose reduction by 8.8%. Negative outcomes were recorded in 32% of the participants (regardless of whether metformin or insulin was administered), being highlighted: neonatal hypoglycemia (<2.6 mmol/L), respiratory distress, need for phototherapy, Apgar score at 5 minutes <7 or preterm birth. Severe hypoglycemia rates (<1.6 mmol/L) were reduced in the metformin group compared to the insulin group. Maternal weight gain to term was significantly lower in women taking metformin than in insulin-treated group (0.4±2.9 kg in the metformin group and 2±3.3 kg in the insulin group; p<0.001)(22).
Balani et al. compared 100 women with gestational diabetes treated with metformin and 100 treated with insulin. The incidence of gestational hypertension, preeclampsia and caesarean section was similar, but the mean increase in maternal weight at term was significantly lower in the metformin group. Pregnant women on metformin treatment had a lower incidence of prematurity, neonatal jaundice, with an overall improvement in neonatal morbidity compared to pregnant women treated with insulin, but no differences in fetal macrosomia were noted(23).
In another study, Balani et al. looked at women with gestational diabetes and metformin treatment compared to women with gestational diabetes who were on diet alone. In the metformin-treated group, there was a tendency to decrease fetal macrosomia and decrease the gestational age, compared to the group that followed only diet(24).
The National Institute for Health and Care Excellence also recommends metformin for women with gestational diabetes if blood glucose targets were not met through changes in diet and exercise for 1-2 weeks, and also recommends insulin instead of metformin for women with gestational diabetes, if metformin is contraindicated or unacceptable(20).
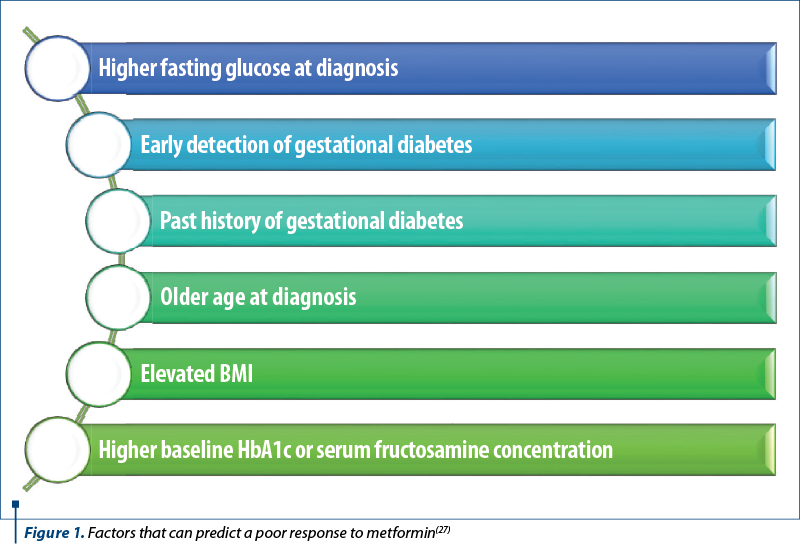
When addressing the response to metformin treatment, several factors that may predict a poor response to metformin (Figure 1) should be considered, which may result in combination insulin therapy(25,26).
Other aspects
Women with polycystic ovarian syndrome may have insulin resistance, and in combination with obesity, the chances of conception and response to fertility treatment decrease and the risk of miscarriage and gestational diabetes increases(28-30). Metformin is used in polycystic ovarian syndrome to induce ovulation, reduce spontaneous abortion rates, prevent fetal growth restrictions, and improve metabolic associations, such as glucose intolerance(31,32).
Metformin is also considered an adjuvant therapy in cancer treatment. The patients with type 2 diabetes on metformin treatment have a lower risk of developing cancer(33,34). However, due to inhibitions caused by metformin, the excessive use in mothers during pregnancy may lead to an increased risk of their children developing metabolic diseases later in life.
Following the risk of malformations in the embryo, metformin administered in doses that stimulated the AMPK activity in the maternal liver did not stimulate the AMPK activity in the embryo, nor did it increase congenital abnormalities. However, metformin stimulated AMPK activity and inhibited the expression of a gene associated with congenital malformations in mouse embryonic stem cells that have been used as an in vitro model to study diabetic embryopathy(12). Pre-implanted human embryos are low in mitochondria and are dependent on anaerobic metabolism, suggesting that early human embryos may be unresponsive to metformin(11).
In order for metformin to affect fetal or placental physiology and development, the cells in these tissues must be able to take over metformin. Because metformin is positively charged at neutral pH, there must be a strong potential of the mitochondrial membrane for it to enter the mitochondrial matrix(35,36). Human placentas express several OCT isoforms, and metformin may indirectly affect the development of the fetus, for example, by modified nutrient administration or placental growth. However, this requires further investigations(9,37). Eyal et al. estimated that the daily intake of metformin transferred through breast milk to infants was 0.13-1.28 mg(14). Metformin reaches into breast milk in a clinically insignificant amount(38).
Conclusions
More and more practitioners consider metformin therapy a good option during pregnancy, because it is generally an effective and well-tolerated agent, with a well-defined mechanism of action. However, there is a need for more detailed studies to determine the degree of fetal exposure and long-term safety following fetal exposure.
Conflicts of interests: The authors declare no conflict of interests.
Bibliografie
- Farrar D, Simmonds M, Bryant M, et al. Hyperglycemia and risk of adverse perinatal outcomes: systematic review and meta-analysis. BMJ. 2016; 354:i4694.
- Falavigna M, Schmidt MI, Trujillo J, et al. Effectiveness of gestational diabetes treatment: a systematic review with quality of evidence assessment. Diabetes Res Clin Pract. 2012; 98(3):396–405.
- Sutherland HW, Stowers JM. Carbohydrate Metabolism in Pregnancy and the Newborn: Incorporating the Proceedings of the International Colloquium at Aberdeen, Scotland, July, 1973. Springer-Verlag London 1989; (1):39-49.
- Coetzee EJ, Jackson WPU. Oral hypoglycaemics in the first trimester and fetal outcome. SA Med J. 1984; 65:635–7.
- Coetzee EJ, Jackson WP. The management of non-insulin-dependent diabetes during pregnancy. Diabetes Res Clin Pract. 1985–1986; 1(5):281–7.
- Stănescu AMA, Grajdeanu IV, Diaconu CC, et al. Evoluţia psoriazisului prenatal, postnatal, afectarea fătului, modificări imune şi hormonale, tratament. Practica Medicală. 2018; 13,1(54):36-40.
- Stănescu AMA, Grăjdeanu IV, Codreanu IF, et al. Dermatological emergencies in neonatology and pediatric practice. Rom J Matern Fetal Neonatal Med. 2018; II(2):80-3.
- Glueck CJ, Goldenberg N, Wang P, et al. Metformin during pregnancy reduces insulin, insulin resistance, insulin secretion, weight, testosterone and development of gestational diabetes: Prospective longitudinal assessment of women with polycystic ovary syndrome from preconception throughout preg. Hum Reprod. 2004; 19:510–21.
- Ahmadimoghaddam D, Zemankova L, Nachtigal P, et al. Organic cation transporter 3 (OCT3/SLC22A3) and multidrug and toxin extrusion 1 (MATE1/SLC47A1) transporter in the placenta and fetal tissues: Expression profile and fetus protective role at different stages of gestation. Biol Reprod. 2013; 88:55.
- Hughes RC, Gardiner SJ, Begg EJ, Zhang M. Effect of pregnancy on the pharmacokinetics of metformin. Diabet Med. 2006; 23(3):323–6.
- Cho YM, Kwon S, Pak YK, et al. Dynamic changes in mitochondrial biogenesis and antioxidant enzymes during the spontaneous differentiation of human embryonic stem cells. Biochem Biophys Res Commun. 2006; 348:1472–78.
- Lee HY, Wei D, Loeken MR. Lack of metformin effect on mouse embryo AMPK activity: Implications for metformin treatment during pregnancy. Diabetes Metab Res Rev. 2014; 30:23–30.
- Wu Y, Viana M, Thirumangalathu S, Loeken MR. AMP-activated protein kinase mediates effects of oxidative stress on embryo gene expression in a mouse model of diabetic embryopathy. Diabetologia. 2012; 55:245–4.
- Eyal S, Easterling TR, Carr D, et al. Pharmacokinetics of metformin during pregnancy. Drug Metab Dispos. 2010; 38(5):833–40.
- Feig DS, Palda VA. Type 2 diabetes in pregnancy: a growing concern. Lancet. 2002; 359(9318):1690–2.
- Abell SK, Nankervis A, Khan KS, Teede HJ. Type 1 and type 2 diabetes preconception and in pregnancy: health impacts, influence of obesity and lifestyle, and principles of management. Semin Reprod Med. 2016; 34(2):110–20.
- Hellmuth E, Damm P, Molsted-Pedersen L. Oral hypoglycaemic agents in 118 diabetic pregnancies. Diabet Med. 2000; 17:507–11.
- Ekpebegh CO, Coetzee EJ, van der Merwe L, Levitt NS. A 10-year retrospective analysis of pregnancy outcome in pregestational type 2 diabetes: comparison of insulin and oral glucose-lowering agents. Diabet Med. 2007; 24:253–8.
- Hughes RC, Rowan JA. Pregnancy in women with type 2 diabetes: who takes metformin and what is the outcome? Diabet Med. 2006; 23:318–22.
- National Institute for Health and Care Excellence (NICE) NICE Guideline (NG3) NICE; London, UK, 2015. Diabetes in pregnancy: Management from preconception to the postnatal period.
- Chiefari E, Arcidiacono B, Foti D, Brunetti A. Gestational diabetes mellitus: An updated overview. J Endocrinol Investig. 2017; 40:899–909.
- Rowan JA, Hague WM, Gao W, et al. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 2008; 358:2003–15.
- Balani J, Hyer SL, Rodin DA, Shehata H. Pregnancy outcomes in women with gestational diabetes treated with metformin or insulin: A case-control study. Diabet Med. 2009; 26:798–802.
- Balani J, Hyer S, Johnson A, Shehata H. Pregnancy outcomes after metformin treatment for gestational diabetes: A case-control study. Obstet Med. 2012; 5:78–82.
- Pantea Stoian A, Mitrofan G, Colceag F, et al. Oxidative stress in diabetes: a model of complex thinking applied in medicine. Rev Chim (Bucharest). 2018; 69(9):2515-9.
- Diaconu C, Bălăceanu A, Bartoş D. Diuretics, first-line antihypertensive agents: are they always safe in the elderly? Rom J Intern Med. 2014; 52(2):87-90.
- Priya G, Kalra S. Metformin in the management of diabetes during pregnancy and lactation. Drugs Context. 2018; 7:212523.
- Balen AH, Anderson RA. Impact of obesity on female reproductive health: British Fertility Society, Policy and Practice Guidelines. Hum Fertil. 2007; 10:195–206.
- Diaconu C, Bălăceanu A, Bartoş D. Venous thromboembolism in pregnant woman – a challenge for the clinician. Central European Journal of Medicine. 2013; 8(5):548-52.
- Diaconu C. Chronic heart failure: diabetes fuels a worse prognosis. Arch Balk Med Union. 2015; 50(1):5-8.
- Kumar P, Khan K. Effects of metformin use in pregnant patients with polycystic ovary syndrome. J Hum Reprod Sci. 2012; 5:166–9.
- Diaconu C. Sindromul metabolic. Ed Medicală, Bucureşti, 2011.
- Gandini S, Puntoni M, Heckman-Stoddard BM, et al. Metformin and cancer risk and mortality: a systematic review and meta-analysis taking into account biases and confounders. Cancer Prev Res (Phila). 2014; 7(9):867–85.
- Totan A, Totan C, Stănescu AMA, et al. Lysyl Oxidase (LOX) – A future new ally on the stage of the fight against cancer. Rev Med Rom. 2019; LXVI(3):214-6.
- Wheaton WW, Weinberg SE, Hamanaka RB et al. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. Elife. 2014; 3:e02242.
- Diaconu C, Nastasă A, Zaki AR, Arsalan M. Type 2 diabetes: a driver for chronic heart failure. The 2nd International conference on interdisciplinary management of diabetes mellitus and its complications – diabetes mellitus as cardiovascular disease, INTERDIAB Proceedings, 2016; 201-10. Ed. Niculescu, Bucureşti.
- Stănescu AMA, Stefani C, Grăjdeanu IV, et al. Asocierea alăptării la sân cu pierderea în greutate. Rev Med Rom. 2019; LXVI(3):226-8.
- Stănescu AMA, Totan A, Mircescu D, et al. Contraindications to breastfeeding – current issues at the border between myth and reality. Modern Med. 2019; 26(3):105-10.
Articole din ediţiile anterioare
Diabetul şi hipertensiunea arterială gestaţională - impactul asupra nou-născuţilor
Diabetul gestaţional este o complicaţie frecventă în ultimii ani, asociată cu greutate excesivă sau obezitate. Pe de altă parte, hipertensiunea ge...
Diabetul gestaţional
Diabetul gestaţional afectează 3-9% din sarcini, implicaţiile acestuia asupra mamei şi copilului fiind uriaşe, întrucât creşte riscul complicaţiilo...
Diabetul gestaţional – o provocare obstetricală, neonatală şi postnatală
In recent decades, the demographics of pregnant women have changed, with an increase of women giving birth at an older age and which, associated wi...
Particularităţi ale evoluţiei materno-fetale la pacientele cu diabet gestaţional
Studiul elaborat a avut ca obiectiv identificarea complicaţiilor, a factorilor de risc şi a altor particularităţi asociate diabetului gestaţional.