Due to the scientific advances of the last decades, cancer patient’s survival has greatly improved, and 70% of the patients now survive more than five years. However, many of the cancer survivors are affected by a constellation of chronic conditions induced by the powerful cancer treatments required to achieve cancer control. The effects of chemotherapy and radiation therapy on the brain are multifactorial and involve acute and sustained insult to neuronal structures essential for normal cognitive processes, as well as for emotional modulation. As a result, the cognitive and emotional deficits associated with cancer treatments are pervasive. The brain-derived neurotrophic factor (BDNF) is an important neuromediator that plays a significant role in developing cancer-related central nervous system complications. Multiple pharmacological strategies have been proven beneficial in raising BDNF levels. Equally important, there are safe and easy to implement dietary and life-style modifications which physicians can recommend, and which can potentially stimulate BDNF synthesis and improve the cancer patient’s cognitive and emotional function and quality of life.
Importanţa factorului neurotrofic derivat din creier în menţinerea sănătăţii cerebrale în timpul şi după tratamentele oncologice
The importance of brain-derived neurotrophic factor in maintaining brain health during and after cancer treatments
First published: 03 aprilie 2018
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/OnHe.42.1.2018.1554
Abstract
Rezumat
Datorită numeroaselor descoperiri ştiinţifice din ultimele decade, supravieţuirea pacienţilor cu cancer a crescut semnificativ, astfel că 70% dintre pacienţii diagnosticaţi trăiesc mai mult de cinci ani. Cu toate acestea, mulţi pacienţi oncologici sunt afectaţi de o constelaţie de condiţii cronice cauzate de tratamentele utilizate pentru controlul cancerului. Efectele citostaticelor şi ale radioterapiei pentru tumorile cerebrale sunt multiple, conducând la numeroase efecte adverse la nivelul structurilor neuronale necesare pentru memorie şi modularea emoţională. Din acest motiv, deficienţele de memorie şi problemele emoţionale sunt observate frecvent. Factorul neurotrofic derivat din creier (BDNF) este un neuromodulator esenţial, cu un rol foarte important în dezvoltarea complicaţiilor la nivelul sistemului nervos central. Intervenţiile farmacologice s-au demonstrat a fi benefice pentru creşterea nivelului de BDNF. De asemenea, medicii pot recomanda modificări privind alimentaţia şi stilul de viaţă, acestea fiind relativ uşor de implementat, dar cu potenţial important de a îmbunătăţi memoria, emoţiile şi calitatea vieţii la pacienţii cu cancer.
Due to the scientific advances of the last decades, cancer patient’s survival has greatly improved, and 70% of the patients now survive more than five years (National Cancer Institute, US). However, many of the cancer survivors are affected by a constellation of chronic conditions induced by the powerful cancer treatments required to achieve disease control. Numerous deficits – such as limb amputations, hair loss or cardiac damage – are easy to identify and to quantify. However, the burden of hidden conditions – such as cognitive and emotional deficits – remains difficult to acknowledge and to measure using objective outcomes.
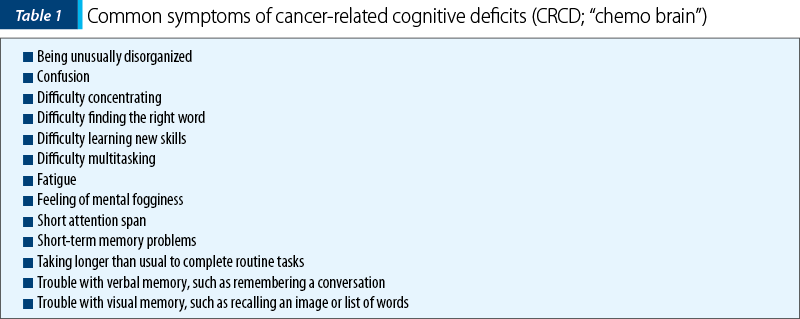
The effects of chemotherapy and radiation therapy on the brain are multifactorial and involve acute and sustained insult to neuronal structures essential for learning and memory, as well as for emotional modulation(1-8). As a result, the cognitive deficits associated with cancer treatments are pervasive, and 15-50% of patients reportedly develop deficits in memory, attention and executive function (Table 1)(9-13). Mood disorders (depression, anxiety) are also extremely common in cancer patients, with the incidence of depression reaching more than 40% of the assessed patients, while the incidence of adjustment disorder was 16%(14,15). Even more significant, the cancer patients diagnosed with a mental illness had a shorter survival than the patients without psychiatric comorbidities(15). The quality of life, job attainment and psychosocial outcomes are also decreased in cancer survivors, many of them reporting forgetfulness and trouble with attention in school or at work(16,17).
The cognitive and emotional deficits seen in cancer patients and cancer survivors are commonly underdiagnosed due to the traditional focus on “fighting the cancer”. Historically, a variety of neuropsychiatric testing measures have been used to define cognitive abilities in cancer patients in clinical trials, but these batteries are difficult to administer and are not commonly used in the clinical practice(9-11,18,19). Computer-based neurocognitive testing is an accessible and objective measure of cognition that, when trended prospectively and longitudinally, can detect and define acute and subacute cognitive changes, and which might be easier to implement in oncology practices in the future(20). Advanced imaging can be also used in the future for diagnosing cancer-induced cognitive deficits. Studies using brain MRI techniques suggested that reduced hippocampal volume are found in chemotherapy treated colon cancer patients(21) and brain tumor patients(22), respectively.
Neurotrophins are part of a class of signaling proteins essential for the development, the survival and the normal function of the nervous system(22). The expression of neurotrophins and their receptors occurs normally during development and in response to stimuli such as tissue injury(23,24). One of the most important neurotrophins is the brain-derived neurotrophic factor (BNDF). BDNF is a growth factor protein encoded by the BDNF gene, broadly expressed in the developing and adult mammalian brain (hippocampus, cortex, basal forebrain)(25,26). BDNF-stimulated intracellular signaling is critical for neuronal survival, morphogenesis and plasticity(27). In hippocampus, BDNF stimulates actin signaling in the dendritic spines and regulates dendritic spine integrity(28), but is also required for neurogenesis(29).
BDNF binds at least two surface receptors (TrkB and LNGFR), which activate multiple intracellular signaling cascades. TrkB (a member of the tyrosine kinase family) is encoded by NTRK2 gene, and its autophosphorylation results from ligand-specific association with BDNF. The BDNF-TrkB pathway plays an important role in short-term memory and neurogenesis. The role of LNGFR (low-affinity nerve growth factor) is still a topic of active research(30).
The roles of BDNF have been implicated in brain aging(31), as well as in the pathophysiology of brain diseases, including Alzheimer, Parkinson and Huntington diseases(32). BDNF replacement enhances neurogenesis and sensorimotor function after stroke(33), improves memory performance in animal models of Alzheimer disease(34) and restores synaptic plasticity in a mouse model of native Huntington disease(35).
BDNF has been proven to be also involved in the pathogenesis of multiple psychiatric illnesses. Higher BDNF levels protect against depression in the elderly(36) and BDNF serum levels can predict the development of depression in stroke patients(37). Increased glucocorticoid production caused by stress is directly linked to decreased BDNF production(38), which in turn further potentiate the depressive symptoms in mice(39). An inverse relation between cortisol and BDNF was also shown in schizophrenia(40) and post-traumatic stress disorder(41). Lower BDNF levels are constantly found in patients with anxiety disorders(42), and were recently recognized as a biomarker for obsessive compulsive disorder(43).
Our published research has shown that chemotherapy induces BDNF downregulation in cultured hippocampal neurons(3). Multiple studies conducted in cancer patients have shown both that BDNF is chronically downregulated in cancer patients(44), and that the lung cancer patients with severely decreased BDNF levels after receiving cancer treatments are more prone to cognitive(45) and mood impairments, as well as to decreased response to chemotherapy (ESMO 2016, Yufeng Wu report). In patients with B-cell non-Hodgkin lymphoma, serum BDNF levels correlate with their ability to perform accurately on cognitive tasks(45). These findings suggest that augmenting BDNF levels through either pharmacological, or non-pharmacological approaches can be potentially beneficial for both normal brain function and response to treatment in cancer patients.
Pharmacological approaches to increase BDNF are already available and include the administration of antidepressants as SSSRIs and of ketamine(46). Furthermore, BDNF activation in the hippocampus is required for the biological activity of the antidepressants in animal models(46). New research also suggests that the augmentation of BDNF levels in hippocampus can be achieved by the administration of positive allosteric modulators of AMPA receptors (ampakines)(47), and that ampakine administration can potentially prevent chemotherapy-induced neuronal damage (data not published).
Interventional approaches to both increase BDNF and to treat medication-refractory depression in selected patients include transcranial magnetic stimulation (TMS)(48) and electroconvulsive therapy (ECT)(49). rTMS upregulates BDNF, as well as improves learning and memory in a preclinical model of vascular dementia(48) and improves motor function in the affected limb, by activating BDNF processing in stroke patients(50). A recent meta-analysis showed that ECT treatment in patients with pharmacological treatment-resistant major depressive disorder significantly increases serum/plasma level of BDNF(51). Though many of these interventions were not tested in cancer patients, they are safe and well tolerated in multiple patients’ populations and can open new areas of potential research in the immediate future.
Multiple nonpharmacologic interventions are also proven to increase BDNF and to help maintain normal cognitive and emotional functions. Environmental enrichment (the creation of a stimulating environment) is proven to increased BDNF, and in turn to increase synaptic plasticity and cognition that potentially slows or reverses cognitive impairment(52,53). Physical exercise also increase the BDNF production and has potential links to improvement in cognitive functions, in young adults(54) and also in elderly population(55). Another potential approach is intermittent fasting, which directly upregulates BDNF expression in the hippocampus and improves synapse associative interactions that might translate into improved learning and memory(56).
Nutritional approaches reported to increase BDNF are numerous, and include fish oil(57), Tualang honey(58), ketogenic diet(59), cocoa(59), high flavonoid fruits and vegetables(59), soy(60), coffee(61) and green tea(61).
Conclusions
BDNF is an important neuromediator that plays a significant role in developing cancer-related central nervous system complications. Pharmacological strategies to increase BDNF expression in cancer patients are still under research, though antidepressants, TMS and ECT have proven benefits in raising BDNF levels. Equally important, there are safe and easy to implement dietary and life-style modifications which physicians can recommend, and which can potentially improve cancer patient’s cognitive and emotional function and quality of life.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
- Dietrich J, Monje M, Wefel J, et al. Clinical patterns and biological correlates of cognitive dysfunction associated with cancer therapy. Oncologist. 2008; 13:1285-95.
- Dietrich J, Han R, Yang Y, et al. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J Biol. 2006; 5:22.
- Andres AL, Gong X, Di K, et al. Low-doses of cisplatin injure hippocampal synapses: a mechanism for ‘chemo’ brain? Exp Neurol. 2014; 255:137-44.
- Rzeski W, Pruskil S, Macke A, et al. Anticancer agents are potent neurotoxins in vitro and in vivo. Ann Neurol. 2004; 56:351-60.
- Seigers R, Schagen SB, Beerling W, et al. Long-lasting suppression of hippocampal cell proliferation and impaired cognitive performance by methotrexate in the rat. Behav Brain Res. 2008; 186:168-75.
- Seigers R, Fardell JE. Neurobiological basis of chemotherapy-induced cognitive impairment: a review of rodent research. Neurosci Biobehav Rev. 2011; 35:729-41.
- Fardell JE, Vardy J, Logge W, et al. Single high dose treatment with methotrexate causes long-lasting cognitive dysfunction in laboratory rodents. Pharmacol Biochem Behav. 2010; 97:333-9.
- Egeland M, Guinaudie C, Du Preez A, et al. Depletion of adult neurogenesis using the chemotherapy drug temozolomide in mice induces behavioural and biological changes relevant to depression. Trans. Psychiatry. 2017; Apr 25;7(4):e1101.
- Kadan-Lottick NS, Zeltzer LK, Liu Q, et al. Neurocognitive functioning in adult survivors of childhood non-central nervous system cancers. J Natl Cancer Inst. 2010; 102:881-93.
- Conklin HM, Krull KR, Reddick WE, et al. Cognitive outcomes following contemporary treatment without cranial irradiation for childhood acute lymphoblastic leukemia. J Natl Cancer Inst. 2010; 104:1386-95.
- Krull KR, Brinkman TM, Li C, et al. Neurocognitive outcomes decades after treatment for childhood acute lymphoblastic leukemia: a report from the St. Jude lifetime cohort study. J Clin Oncol. 2013; 31:4407-15.
- Vardy J, Tannock I. Cognitive function after chemotherapy in adults with solid tumours. Crit Rev Oncol Hematol. 2007; 63:183-202.
- Vannorsdall TD. Cognitive Changes Related to Cancer Therapy. Med Clin North Am. 2017; 101:1115-1134.
- Rooney AG, Carson A, Grant R. Depression in cerebral glioma patients: a systematic review of observational studies. J Natl Cancer Inst. 2011; 103:61-76.
- Chan CM, Wan Ahmad WA, Yusof MM, et al. Effects of depression and anxiety on mortality in a mixed cancer group: a longitudinal approach using standardised diagnostic interviews. Psychooncology. 2015; 24:718-25.
- Parsons HM, Harlan LC, Lynch CF, et al. Impact of cancer on work and education among adolescent and young adult cancer survivors. J Clin Oncol. 2012; 30:2393-400.
- Edelstein K, Coate L, Massey C, et al. Illness intrusiveness and subjective well-being in patients with glioblastoma. J Neurooncol. 2016; 126:127-35.
- Duffner PK, Armstrong FD, Chen L, et al. Neurocognitive and neuroradiologic central nervous system late effects in children treated on Pediatric Oncology Group (POG) P9605 (standard risk) and P9201 (lesser risk) acute lymphoblastic leukemia protocols (ACCL0131): a methotrexate consequence? A report from the Children’s Oncology Group. J Pediatr Hematol Oncol. 2014; 36:8-15.
- Langer T, Martus P, Ottensmeier H, et al. CNS late-effects after ALL therapy in childhood. Part III: neuropsychological performance in long-term survivors of childhood ALL: impairments of concentration, attention, and memory. Med Pediatr Oncol. 2002; 38:320-8.
- Lim YY, Ellis KA, Harrington K, et al. Use of the CogState Brief Battery in the assessment of Alzheimer’s disease related cognitive impairment in the Australian Imaging, Biomarkers and Lifestyle (AIBL) study. J Clin Exp Neuropsychol. 2012; 34:345-58.
- Schneiderman B. Hippocampal volumes smaller in chemotherapy patients. Lancet Oncol. 2004; 5:202.
- Nolen SC, Lee B, Shantharam S, et al. The effects of sequential treatments on hippocampal volumes in malignant glioma patients. J Neurooncol. 2016; 129:433-441.
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001; 24:677-736.
- Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci. 2001; 24:1217-81.
- Mandel AL, Ozdener H, Utermohlen V. Identification of pro- and mature brain-derived neurotrophic factor in human saliva. Arch Oral Biol. 2009; 54:689-95.
- Wetmore C, Ernfors P, Persson H, et al. Localization of brain-derived neurotrophic factor mRNA to neurons in the brain by in situ hybridization. Exp Neurol. 1990; 109:141-52.
- Lipsky RH, Marini AM. Brain-derived neurotrophic factor in neuronal survival and behavior-related plasticity. Ann N Y Acad Sci. 2007; 1122:130-43.
- An JJ, Gharami K, Liao GY, et al. Distinct role of long 3’ UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell. 2008; 134:175-87.
- Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002; 82:1367-75.
- Bartkowska K, Paquin A, Gauthier AS, et al. Trk signaling regulates neural precursor cell proliferation and differentiation during cortical development. Development. 2007; 134:4369-80.
- Kirk IE, Destiny LM, Kathryn AR. The Aging Hippocampus: Interactions between Exercise, Depression, and BDNF. The Neuroscientist. 2011; 18:82-97.
- Mattson MP. Glutamate and neurotrophic factors in neuronal plasticity and disease. Ann NY Acad Sci. 2008; 1144:97-112.
- Schabitz WR, Steigleder T, Cooper-Kuhn CM, et al. Intravenous brain-derived neurotrophic factor enhances poststroke sensorimotor recovery and stimulates neurogenesis. Stroke. 2007; 38:2165-72.
- Blurton-Jones M, Kitazawa M, Martinez-Coria H, et al. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci USA. 2009; 106:13594-9.
- Lynch G, Kramar EA, Rex CS, et al. Brain-derived neurotrophic factor restores synaptic plasticity in a knock-in mouse model of Huntington’s disease. J Neurosci. 2007; 27:4424-34.
- Kanellopoulos D, Gunning FM, Morimoto SS, et al. Hippocampal Volumes and the Brain-Derived Neurotrophic Factor val66met Polymorphism in Geriatric Major Depression. The American Journal of Geriatric Psychiatry. 2011; 19:13-22.
- Li J, Zhao Y-D, Zeng J-W, et al. Serum brain-derived neurotrophic factor levels in post-stroke depression. Journal of Affective Disorders. 2014; 168:373-379.
- Naveen GH, Varambally S, Thirthalli J, et al. Serum cortisol and BDNF in patients with major depression - effect of yoga. Int Rev Psychiatry. 2016; 28:273-8.
- Curto M, Martocchia A, Ferracuti S, et al. Increased Total Urinary Cortisol (tUC) and Serum Brain-derived Neurotrophic Factor (BDNF) Ratio in Alzheimer Disease (AD)-affected Patients. Alzheimer Dis Assoc Disord. 2017; 31:173-176.
- Issa G, Wilson C, Terry AV Jr., et al. An inverse relationship between cortisol and BDNF levels in schizophrenia: data from human postmortem and animal studies. Neurobiol Dis. 2010; 39:327-33.
- Simsek S, Uysal C, Kaplan I, et al. BDNF and cortisol levels in children with or without post-traumatic stress disorder after sustaining sexual abuse. Psychoneuroendocrinology. 2015; 56:45-51.
- Suliman S, Hemmings S, Seedat S. Brain-Derived Neurotrophic Factor (BDNF) protein levels in anxiety disorders: systematic review and meta-regression analysis. Frontiers in Integrative Neuroscience. 2013; 7.
- Oliveira-Maia AJ, Castro-Rodrigues P. Brain-derived neurotrophic factor: a biomarker for obsessive-compulsive disorder? Frontiers in Neuroscience. 2015; 9.
- Brum C, Stertz L, Borba E, et al. Association of serum brain-derived neurotrophic factor (BDNF) and tumor necrosis factor-alpha (TNF-alpha) with diagnosis of delirium in oncology inpatients. Rev Bras Psiquiatr. 2015; 37:197-202.
- Zimmer P, Mierau A, Bloch W, et al. Post-chemotherapy cognitive impairment in patients with B-cell non-Hodgkin lymphoma: a first comprehensive approach to determine cognitive impairments after treatment with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone or rituximab and bendamustine. Leuk Lymphoma. 2014; 1-6.
- Björkholm C, Monteggia LM. BDNF – a key transducer of antidepressant effects. Neuropharmacology. 2016; 102:72-79.
- Lauterborn JC, Lynch G, Vanderklish P, et al. Positive Modulation of AMPA Receptors Increases Neurotrophin Expression by Hippocampal and Cortical Neurons. J. Neurosci. 2000; 20:8-21.
- Zhang N, Xing M, Wang Y, et al. Repetitive transcranial magnetic stimulation enhances spatial learning and synaptic plasticity via the VEGF and BDNF-NMDAR pathways in a rat model of vascular dementia. Neuroscience. 2015; 311:284-91.
- Salehi I, Hosseini SM, Haghighi M, et al. Electroconvulsive therapy (ECT) and aerobic exercise training (AET) increased plasma BDNF and ameliorated depressive symptoms in patients suffering from major depressive disorder. J Psychiatr Res. 2016; 76:1-8.
- Niimi M, Hashimoto K, Kakuda W, et al. Role of Brain-Derived Neurotrophic Factor in Beneficial Effects of Repetitive Transcranial Magnetic Stimulation for Upper Limb Hemiparesis after Stroke. PLoS One. 2016; 11:e0152241.
- Rocha RB, Dondossola ER, Grande AJ, et al. Increased BDNF levels after electroconvulsive therapy in patients with major depressive disorder: A meta-analysis study. J Psychiatr Res. 2016; 83:47-53.
- Gobbo OL, O’Mara SM. Impact of enriched-environment housing on brain-derived neurotrophic factor and on cognitive performance after a transient global ischemia. Behav Brain Res. 2004; 152:231-41.
- Novkovic T, Mittmann T, Manahan-Vaughan D. BDNF contributes to the facilitation of hippocampal synaptic plasticity and learning enabled by environmental enrichment. Hippocampus. 2015; 25:1-15.
- Hotting K, Schickert N, Kaiser J, et al. The Effects of Acute Physical Exercise on Memory, Peripheral BDNF, and Cortisol in Young Adults. Neural Plast. 2016; 6860573.
- Hakansson K, Ledreux A, Daffner K, et al. BDNF Responses in Healthy Older Persons to 35 Minutes of Physical Exercise, Cognitive Training, and Mindfulness: Associations with Working Memory Function. J Alzheimers Dis. 2017; 55:645-657.
- Dasgupta A, Kim J, Manakkadan A, et al. Intermittent fasting promotes prolonged associative interactions during synaptic tagging/capture by altering the metaplastic properties of the CA1 hippocampal neurons. Neurobiol Learn Mem. 2017; Dec 19. pii: S1074-7427(17)30206-X.
- Wu A, Ying Z, Gomez-Pinilla F. Dietary omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J Neurotrauma. 2004; 21:1457-67.
- Othman Z, Zakaria R, Hussain NHN, et al. Potential Role of Honey in Learning and Memory. Med Sci (Basel). 2015; 3:3-15.
- Brownlow ML, Jung SH, Moore RJ, et al. Nutritional Ketosis Affects Metabolism and Behavior in Sprague-Dawley Rats in Both Control and Chronic Stress Environments. Front Mol Neurosci. 2017; 10:129.
- Katayama S, Imai R, Sugiyama H, et al. Oral administration of soy peptides suppresses cognitive decline by induction of neurotrophic factors in SAMP8 mice. J Agric Food Chem. 2014; 62:3563-9.
- Sangiovanni E, Brivio P, Dell’Agli M, et al. Botanicals as Modulators of Neuroplasticity: Focus on BDNF. Neural Plasticity. 2017; 5965371.
Articole din ediţiile anterioare
Terapia anti HER-2 în cancerul sânului, o scurtă privire asupra opţiunilor terapeutice disponibile
În această prezentare voi detalia noile terapii disponibile pentru tratarea cancerului de sân cu supraexpresie HER-2 (o oncogenă a familiei EGFR - ...
Cancerul: imagine de ansamblu. Văzând pădurea dincolo de copaci
Vă prezentăm aici pentru prima dată conceptul de sistem al cancerului. Acest sistem este format din mai multe ţesuturi canceroase separate geografi...