Din aceeași categorie
Introduction
Renal cancer represents 4.3% of all adult neoplasiae according to Globocan estimation, representing the 7th cause of cancer in men and 9th cause of cancer in women. Annually, in the world are diagnosed 200,000 new cases of renal cancer and there are 100,000 deaths. This disease affects predominantly males in 2/1 rapport with women. Median age at diagnostic is 60 years.
In Romania renal cancer is situated on the 12th place of all cancers with an estimated incidence of 7.7/100,000.
Treatment schemas have suffered major changes in 2005/2007 when FDA and EMA approved a new series of molecules that target angiogenesis in advanced or metastatic renal cancer.
Since then, the number of new molecules registered in renal cancer treatment raised to 7. This fact is observed in survival benefit with an increased median survival from 26.4 months in 2006 to 32 months in 2014 as reported by JRMotzer in New England Journal in 2007 and at ASCO 2013.
First therapeutic classes used as antiangiogenic treatment were:
1. Anti VEGF - Bevacizumab - approved by EMA in December 2007 and by FDA in July 2009
2. Anti VGEFR:
a. Sunitinib, approved by EMA in August 2009 and by FDA in February 2007
b. Sorafenib, approved by EMA in December 2009 and by FDA in November 2007
c. Pazopanib, approved by EMA in July 2010 and by FDA in October 2009
d. Axitinib, approved by EMA in August 2012 and by FDA in January 2012.
3. MTor Inhibitors:
a. Temsirolimus approved by EMA in September 2009 and by FDA in May 2007
b. Everolimus approved by EMA in September 2009 and by FDA in May 2009.
Vascular endothelial growth factors (VEGFs) play a crucial role in tumour angiogenesis and development in renal cell carcinoma (RCC)(1,2). Advances in the understanding of RCC tumour biology have enabled the successful clinical development of molecular targeted therapies. Inhibition of angiogenesis by targeting the VEGF or mammalian target of rapamycin (mTOR) pathways has been shown to be an effective treatment for metastatic RCC (mRCC), improving overall response rates and progression-free survival (PFS) rates in mRCC compared with interferon (IFN)-based therapies(3).
In a Phase III clinical trial, first-line treatment with the VEGF receptor (VEGFR) tyrosine kinase inhibitor (TKI) sunitinib was shown to extend PFS (11 months vs 5 months) and lead to a higher response rate (31% vs 6%) compared with IFN-a(4).
In a Phase III trial, the mTOR inhibitor everolimus was shown to significantly extend PFS vs placebo (4.9 vs 1.9 months; p<0.0001) as second-line treatment in patients with mRCC who had progressed following treatment with sunitinib, sorafenib or both(5).
In a Phase III trial of second-line axitinib compared with sorafenib, PFS was 8.3 months (95% CI, 6.7-9.2) with axitinib vs 5.7 months (4.7-6.5) with sorafenib (HR, 0.656; 95% CI, 0.552-0.779; one-sided p<0.0001)(6).
The latest guidelines recommend sunitinib for first‑line therapy for mRCC.(7-9) Second-line therapies that are recommended after progression following treatment with a TKI include axitinib (TKI) and everolimus (mTOR inhibitor)(8).
Romania was among the first countries that reimbursed 4 targeted molecules in renal cancer: for patients with good and intermediary prognostic - Sunitinib, Sorafenib and Bevacizumab with Interferon and for patients with poor prognostic - Temsirolimus.
Since 2008 till present EMA and FDA approved other molecules for treatment, but Romanian reimbursement list was not updated, so treatments had to be done with those molecules.
Here we present a case of a patient with metastatic clear cell RCC with a treatment effect following sequential VEGF and mTOR inhibitor treatment due to accessibility to mTOR inhibitor.
Patient presentation
January 2011: A 50-year-old man, with no previous relevant personal or family medical history, presented with right lumbar pain, which was resistant to analgesia.
February 2011:
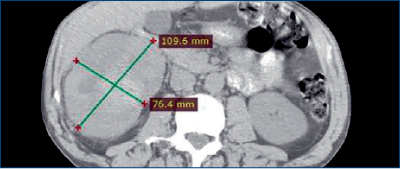
- Abdominal CT scan revealed a mass in the right kidney (109 x 76 mm) (Figure 1) and thoracic CT scans showed no metastases.
- The patient consequently underwent a radical nephrectomy. Subsequent histology revealed this was a clear cell RCC (pT3apN0), with peri-renal fat extension
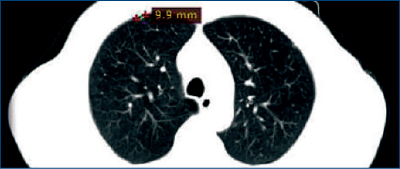
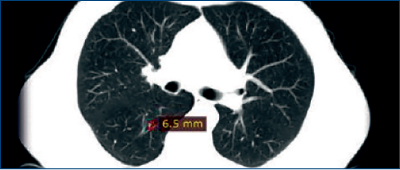
January 2012: A routine CT scan revealed pulmonary metastases (Figure 2).
March 2012: The patient began sunitinib (50 mg PO QD, 4 weeks on/2 weeks off) after obtaining approval of Romanian Insurance System.
September 2012: No change in the size of the pulmonary metastases was observed on routine CT scans
- The patient was deemed to have stable disease (RECIST 1.1).
- The patient did not experience any side effects with sunitinib treatment.
January 2013:
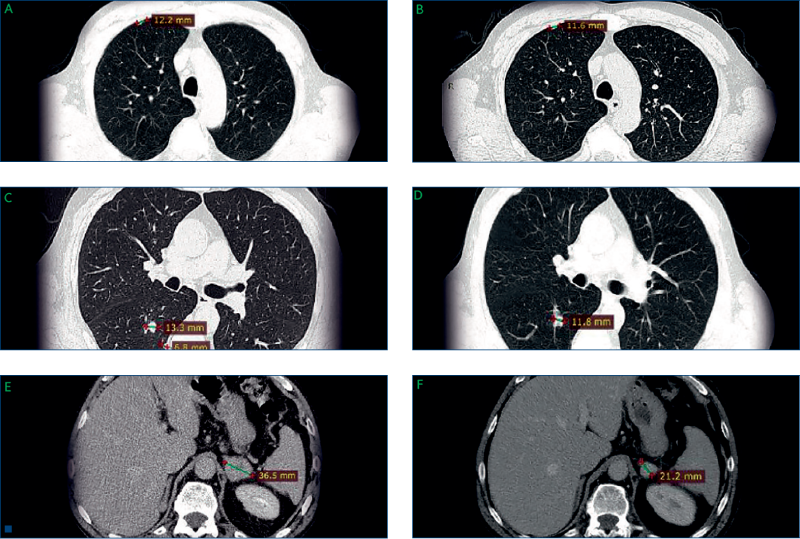
- Routine CT demonstrated that the pulmonary metastases had increased in size (Figures 3A and C), and a further metastasis was observed in the left adrenal gland (Figure 3E).
- Sunitinib treatment was stopped after six cycles, and second-line treatment with temsirolimus was prescribed*.
February 2013: The patient began temsirolimus (25 mg IV weekly) treatment.
May 2014:
- Temsirolimus treatment is ongoing and the patient has not experienced any side effects.
- Routine CT scan revealed shrinkage of the lung and the adrenal metastases (Figure 3B and D, and Figure 3F, respectively), and the patient was considered to have a partial response (by RECIST criteria).
Conclusions
Here we supply case details of a patient with mRCC treated with sunitinib, who had a PFS of approximately 9 months, similar to the PFS observed in clinical trials(4). Sunitinib was also well tolerated by this patient.
Temsirolimus, an mTOR inhibitor, is currently only approved for the first-line treatment of mRCC patients with poor prognosis(10).
Here we demonstrate a treatment effect of second‑line temsirolimus in a patient with mRCC, which suggests that this may be an option for treatment if everolimus is unavailable.
medicamentetratament cancer renalOrdin de ministru pentru includerea a 35 de medicamente pe lista cu compensate
Pacienții vor avea acces la noi tratamente compensate.
...OMS actualizat lista medicamentelor esențiale pentru adulți și copii
Organizația Mondială a Sănătății a adăugat pe lista medicamentelor esențiale mai multe produse medicamentoase pentru tratamentul cancerului și al diabetului zaharat.
...Numele ANMDMR, folosit în reclame înșelătoare
ANMDMR recomandă tuturor să se informeze exclusiv din surse oficiale și să solicite opinia unui profesionist.
...

