ToRCH – toxoplasmosis, rubella, cytomegalovirus (CMV) and herpes simplex virus (HSV) – are the most common causes of congenital infections worldwide. Congenital infections may have various outcomes, from intrauterine death to clinically asymptomatic infections. The treatments of ToRCH infections in pregnancy are directed to reducing maternal symptoms and preventing maternal-to-child transmission of pathogens. The early recognition and the prenatal approach for congenital infections are key aspects in the management of ToRCH infections.
Infecţiile ToRCH – toxoplasmoza, rubeola, citomegalovirusul şi virusul herpes simplex - abordare şi management
ToRCH infections in pregnancy: approach and management
First published: 17 decembrie 2019
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/ObsGin.67.4.2019.2759
Abstract
Rezumat
ToRCH – toxoplasmoza, rubeola, citomegalovirusul (CMV) şi virusul herpes simplex (HSV) – sunt cele mai frecvente cauze ale infecţiilor congenitale la nivel global. Infecţiile congenitale pot avea diverse consecinţe, de la moarte intrauterină la infecţii asimptomatice clinic. Tratamentele infecţiilor cu ToRCH în sarcină sunt orientate către reducerea simptomelor materne şi prevenirea transmiterii patogenilor de la mamă la copil. Recunoaşterea timpurie şi abordarea prenatală a infecţiilor congenitale sunt aspecte-cheie în gestionarea infecţiilor cu ToRCH.
Introduction
ToRCH – toxoplasmosis, rubella, cytomegalovirus (CMV) and herpes simplex virus (HSV) – are the most common causes of congenital infections worldwide(1), and may result in lengthy hospitalization, prolonged suffering, lifelong disability and even death. The modes of transmission are similarly varied, including transplacental spread, intrauterine contamination, and exposure at the time of delivery and beyond(2).
Pregnant women present an increased severity of infections with some pathogens, including ToRCH infections, and immune adaptations are required to accommodate the fetus(1).
This review presents options for the evaluation and management of ToRCH risks during pregnancy.
Toxoplasma gondii infection
Toxoplasmosis is caused by the infection with Toxoplasma gondii, an obligate intracellular parasite which has the capacity to cross the placenta and infect the fetus when pregnant women are primarily infected during gestation. Acute toxoplasmosis during pregnancy is detrimental to the developing fetus(3). Chorioretinitis, hydrocephalus, intracranial calcifications and convulsions are the typical presentation of classic congenital toxoplasmosis(4). This tropism for the central nervous system (CNS) underlies the devastating disease which T. gondii causes in those with deficient immune responses – e.g., developing fetuses or AIDS patients(5).
The serologic screening should be offered only to pregnant women considered to be at risk for primary Toxoplasma gondii infection, with ultrasound findings including but not limited to intracranial calcification, microcephaly, hydrocephalus, ascites, hepatosplenomegaly, or severe intrauterine growth restriction(6).
No IgG and IgM antibodies found before or early in pregnancy shows that a previous contact with the parasite is unlikely, and identifies women at risk of acquiring the infection during pregnancy. High IgM and IgG antibody levels suggest an acute infection within the previous three months. IgA antibodies are considered to be a marker of acute infection, which are produced earlier than IgM, and may persist for several months. The shorter period of IgE may give a greater indication of current infection. The presence of IgG antibodies suggests the occurrence of infection, but does not provide any information about the timing of infection(7). The avidity test can help determine the timing of Toxoplasma gondii infection in relation to pregnancy. The test should be performed, in view of the possibility of antenatal treatment of the fetus(8).
If maternal infection has been confirmed but the fetus is not yet known to be infected, spiramycin is prescribed (it seems to control placenta infection) at a dosage of 1 g per day orally every 8 hours for the duration of pregnancy(9). A combination of pyrimethamine, sulfadiazine and folinic acid should be offered as treatment for women in whom fetal infection has been confirmed or is highly suspected, usually by a positive amniotic fluid polymerase chain reaction(6).
Recommendations made according to guidelines and triple drug treatment: spiramycin 3 g (=9 MIU) per day orally in three divided doses, until the end of the first trimester of pregnancy (it does not cross the fetoplacental barrier), from the 16th or the 18th week of pregnancy. 1. Sulfadiazine: 50 mg/kg/day to 4 g orally, in four divided doses. 2. Pyrimethamine: 50 mg on the first day, 25 mg on the following days, orally as a single dose (potential teratogenic effect). 3. Folinic acid: 10 to 15 mg/day, orally (the prevention of hematopoietic inhibition). Sulfadiazine-pyrimethamine combination with folinic acid alternates with spiramycin in three or four weekly cycles until delivery is given. Weekly blood counts are needed to monitor hematopoiesis(8,10).
The treatment of acute infection during pregnancy has been associated with an approximately 50% reduction in fetal infection(11).
Early in pregnancy transmission rates from mother to fetus tend to be low, while fetal disease severity is highest. In contrast, maternal infection in the third trimester often results in asymptomatic newborns(8,9).
The prevalence of toxoplasmosis is decreasing markedly in Europe, weakening the effect of preventive measures and questioning the rationale for screening(12).
Congenital rubella
Maternal rubella infection in the first trimester of pregnancy results in fetal infection rates of 80% to 100%(13). The hallmark clinical features of congenital rubella syndrome (CRS) are cataracts, sensorineural hearing loss, and congenital heart disease, consisting of patent ductus arteriosus or septal defects. Infections prior to the 16th week produce fetal loss, cataracts and/or heart disease(14). By 16 weeks of gestation, the fetal infection rate drops to 10% or 20%, but it rises again to 60% or higher after 30 weeks of gestation(13).
Rubella infection may be identified by the detection of virus by polymerase chain reaction (PCR) (prenatally), hemagglutination inhibition (HAI), haemolysis in gel test (HIG), and the detection of antibodies by EIA or ELISA. The measurement of antibodies in the serum is important for the determination of the immune status, especially the screening of young women in prenatal care – Table 1(15).

Preventing these adverse pregnancy outcomes is the focus of rubella vaccination programs. The Global Vaccine Action Plan 2011-2020 (GVAP), endorsed by the World Health Assembly in 2012, includes goals to eliminate rubella in at least five of the six WHO regions by 2020. In the European Region, 33 (62%) of 53 countries were declared free of endemic rubella virus transmission in 2016(16).
Cytomegalovirus (CMV)
The prevalence of congenital CMV infection vary widely depending on the characteristics of maternal populations, such as age and maternal CMV seroprevalence. However, a reasonable estimate of the overall birth prevalence of congenital CMV infection is about 4 to 5/1,000 live births. Sensorineural hearing loss is by far the most frequent long-term sequela in congenital CMV infection and occurs in 8-15% of infected newborns(17). Collected data suggest that a cytomegalovirus infection can be severe only when the virus hits the fetus in the embryonic or early fetal period. Congenital CMV infection may have various outcomes, from intrauterine death to clinically asymptomatic infections(18).
Primary infections in the mother have a much greater clinical impact on the fetus than reactivated infections or exogenous reinfections. An optimal approach to CMV infection in pregnancy should probably be primary prevention in seronegative pregnant women, identified by pre-pregnancy or early pregnancy screening(19).
Proposed management for CMV infected fetus – Figure 1(20)
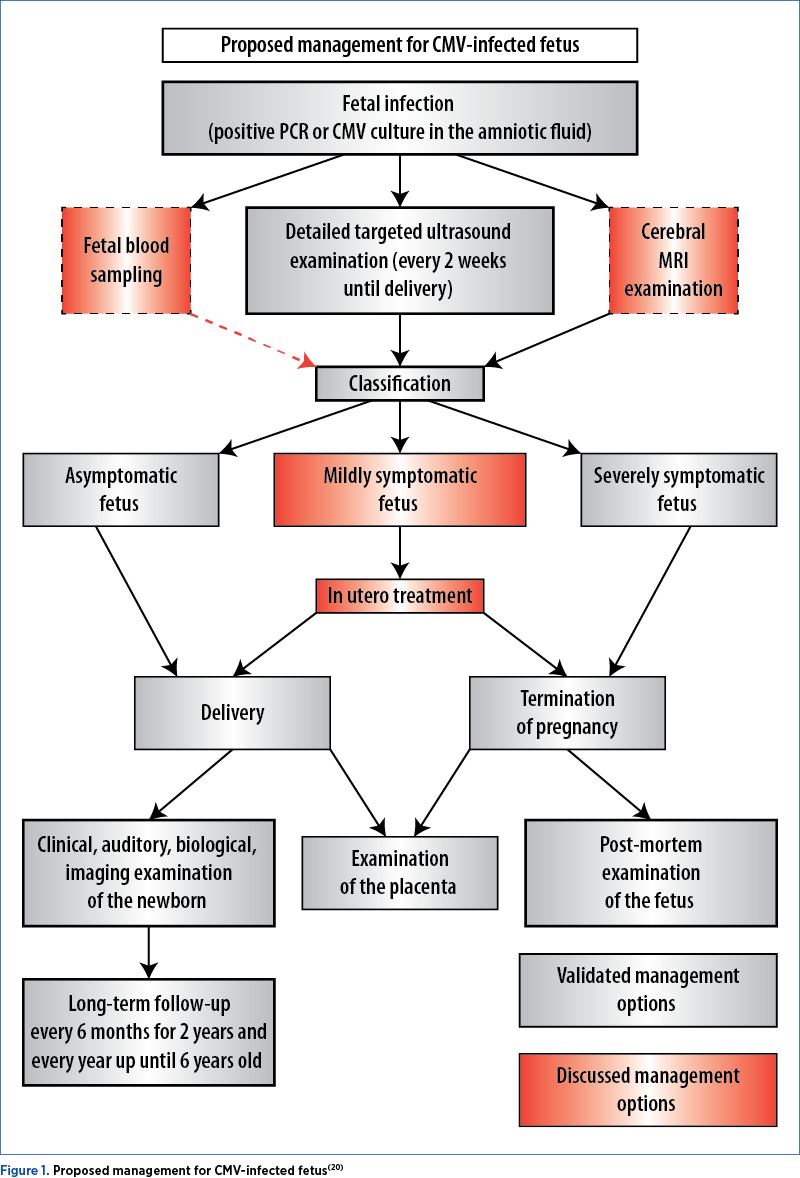
Laboratory testing for CMV is needed in particular clinical conditions, such as abnormal ultrasound findings(21). Hematological disorders such as neutropenia and thrombocytopenia are rare in CMV-infected fetuses, although CMV is occasionally associated with mild fetal anemia(22). In the context of a proven intrauterine infection, the negative predictive value of ultrasound coupled with fetal brain MRI is over 85%(23).
The determination of CMV antibody avidity carried out before weeks 12-16 of gestation helps to interpret a positive result for CMV IgM, therefore it is a helpful tool to identify all pregnant women who may give birth to an infected newborn (100% sensitivity). If the IgG avidity index is determined later during pregnancy (after 18-20 weeks of gestation), the sensitivity is drastically reduced (62.5%). A high avidity index during the first 12-16 weeks of gestation can be considered a good indicator of past infection(24).
The diagnosis of maternal primary infection remains tricky because the interpretation of IgG avidity test may be difficult(23). Amniocentesis with amplification of the viral genome in the amniotic fluid by PCR is reliable for the diagnosis of fetal infection, but there are two factors to take into account: gestational age and the time interval (6-8 weeks) between maternal seroconversion and amniocentesis(25).
The treatment of symptomatic non-pregnant woman with antiviral agents results in favorable clinical outcomes. There is also some evidence that valaciclovir (8 g daily; 2 g four times a day) treatment of mothers carrying an infected fetus from the time of prenatal diagnosis (range 22-34 weeks) up until delivery is feasible, safe and might be effective.
Symptomatic fetuses were defined by the presence of measurable extracerebral or mild cerebral ultrasound symptoms(26). In the case of severely symptomatic fetuses with severe ultrasound findings (hydrops, microcephaly, ventriculomegaly measuring >15 mm, white matter abnormalities and parenchymatous cavitations, intracerebral hemorrhages, delayed cortical development etc.) associated with thrombocytopenia, the termination of pregnancy is found to be acceptable at maternal request(20).
In contrast to the success of rubella vaccines that induce antibody responses similar to those measured following the infection with wild rubella virus, research is ongoing to clarify the role of treatments for cytomegalovirus(17).
Herpes simplex virus (HSV)
Herpes simplex viruses types 1 and 2 are simplexviruses in the Alphaherpesvirinae subfamily and important pathogens with a latent carrier restricted to humans. The virus evades the immune response within the sacral ganglion and cannot be eliminated. HSV at the time of labor and delivery causes neonatal herpes, a rare infection, long-term neurologic impairment and high mortality rates in neonates. Genital HSV contributes to increased risk of HIV acquisition and transmission, and pregnant women should therefore receive the highest priority for HIV prevention(27,28).
Herpes simplex virus type 2 (HSV-2) is the most prevalent sexually transmitted infection worldwide, with a seroprevalence ranging from ~20% among adults in the general population of the United States of Amrica and parts of Western Europe to 50-70% in sub-Saharan Africa(29).
The seroprevalence in women is approximately twofold higher than in men, and seroprevalence increases with age and the number of sexual partners. In USA and northern Europe, the changing epidemiology of genital herpes has been associated with HSV-1, now causing the majority of first-episode genital herpes in women under the age of 26(27,30).
First-episode primary genital herpes is more severe than first-episode non-primary genital herpes. In general, non-primary HSV-1 is rare. Serologic testing must be done near the onset of symptoms prior to seroconversion (negative HSV-1, HSV-2 serology) to confirm the primary diagnosis of HSV-2 (genital HSV culture/PCR HSV-2 +)(30,31).
Intrauterine HSV infection of the fetus occurs in about 5% of cases, unlike the peripartal and postpartal infections that are more common(21). Primary herpes may be associated with early miscarriage(32). Despite the low incidence during the intrauterine period, they are significant because of potentially fatal consequences such as fetal death or neurological disorders, the development of encephalomalacia (especially in temporal regions), encephalitis, intracranial calcification and microcephaly, microphthalmia and optic nerve atrophy(21).
Recommended regimens in non-pregnant women: aciclovir 400 mg orally, three times a day, for 7-10 days, or aciclovir 200 mg orally, five times a day, for 7-10 days, or famciclovir 250 mg orally, three times a day, for 7-10 days, or valacyclovir 1 g orally, twice a day, for 7-10 days. Unlike famciclovir and valacyclovir, aciclovir has poor oral bioavailability; frequent high doses are needed for adequate antiviral activity(33).
The use of continuous aciclovir to suppress recurrent herpes throughout pregnancy is inadvisable(32). The suppressive therapy for the prevention of HSV-2 transmission from HIV/HSV-2 coinfected individuals is not effective(31).
Caesarean section should be performed if an active primary or recurrent herpes outbreak is suspected at delivery, in order to prevent neonatal transmission. Current guidance from the Royal College of Obstetricians and Gynaecologists and The Centre for Disease Control is to treat at the time of infection for 7-10 days with 400 mg oral aciclovir, three times a day, followed by a suppressive dose of 400 mg of aciclovir, once a day, from 36 weeks of gestation until delivery(34,35).
Conclusions
Congenital infections with toxoplasmosis, rubella, cytomegalovirus and herpes simplex cause variable degrees of cerebral abnormality and fetal damage, with worldwide distribution and a high clinical importance(36).
Since guidelines on prenatal management have cautious approach, priorities for research include: a) improving estimates regarding the immunologic changes that occur as pregnancy progresses and the interplay of infection, pregnancy, and the fetus and placenta; b) concerns about false-positive test results to establish the acute (primary) infection and distinguish it from past (chronic) infection, in evaluating the risk of fetal transmission; c) determining the applicability of national screening programs; d) potential risks associated with ToRCH coinfections, which may be able to lead to more complicated pregnancy outcomes(1,9,11,30).
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
2. Frehm E, Harris MH. Congenital and Perinatal Infections. Comprehensive Pediatric Hospital Medicine. Elsevier. 2007.
3. Hampton MM. Congenital Toxoplasmosis: A Review. Neonatal Netw. 2015;34(5):274-8, doi: 10.1891/0730-0832.34.5.274.
4. Martin S. Congenital Toxoplasmosis. Neonatal Network. 2001 Jun; 20 (4), doi: 10.1891/0730-0832.20.4.23.
5. Mendez OA, Koshy AA. Toxoplasma gondii: Entry, association, and physiological influence on the central nervous system. PLoS Pathog. 2017;13(7):e1006351, doi: 10.1371/journal.ppat.1006351.
6. Paquet C, Yudin MH. Toxoplasmosis in Pregnancy: Prevention, Screening, and Treatment. J Obstet Gynaecol Can. 2018; 40(8):e687-e693, doi: 10.1016/j.jogc.2018.05.036.
7. Liu Q, Wang ZD, Huang SY, Zhu XQ. Diagnosis of toxoplasmosis and typing of Toxoplasma gondii. Parasit Vectors. 2015; 8:292, doi: 10.1186/s13071-015-0902-6.
8. Lopes FM, Gonçalves DD, Mitsuka-Breganó R, Freire RL, Navarro IT. Toxoplasma gondii infection in pregnancy. Braz J Infect Dis. 2007; 11(5):496-506.
9. Chaudhry SA, Gad N, Koren G. Toxoplasmosis and pregnancy. Can Fam Physician. 2014; 60(4):334-6.
10. Gilbert R, Gras L; European Multicentre Study on Congenital Toxoplasmosis. Effect of timing and type of treatment on the risk of mother to child transmission of Toxoplasma gondii. BJOG. 2003; 110(2):112-20.
11. Lopez A, Dietz VJ, Wilson M, Navin TR, Jones JL. Preventing congenital toxoplasmosis. MMWR Recomm Rep. 2000; 49(RR-2):59-68.
12. Ville Y, Leruez-Ville M. Managing infections in pregnancy. Curr Opin Infect Dis. 2014; 27(3):251-7. doi: 10.1097/QCO.0000000000000066.
13. Maldonado YA. Rubella Virus. Principles and Practice of Pediatric Infectious Diseases (Fourth Edition). Elsevier. 2012.
14. Balejr JF. Rubella. Handbook of Clinical Neurology. Elsevier. 2014.
15. DRG Rubella ELISA. Vers 1.0 2013.
16. Grant GB, Reef SE, Patel M, Knapp JK, Dabbagh A. Progress in Rubella and Congenital Rubella Syndrome Control and Elimination - Worldwide, 2000-2016. MMWR Morb Mortal Wkly Rep. 2017; 66(45):1256-1260, doi: 10.15585/mmwr.mm6645a4.
17. Britt WJ. Congenital Human Cytomegalovirus Infection and the Enigma of Maternal Immunity. J Virol. 2017; 91(15). pii: e02392-16, doi: 10.1128/JVI.02392-16.
18. Faure-Bardon V, et al. Sequelae of congenital cytomegalovirus (cCMV) following maternal primary infection are limited to those acquired in the first trimester of pregnancy. Clin Infect Dis. 2019 Oct 15;69(9):1526-1532.
19. Nigro G, et al. Clinical manifestations and abnormal laboratory findings in pregnant women with primary cytomegalovirus infection. BJOG. 2003; 110(6):572-7.
20. Benoist G, et al. Management of pregnancies with confirmed cytomegalovirus fetal infection. Fetal Diagn Ther. 2013; 33(4):203-14, doi:10.1159/000342752.
21. Amidzic J, Vuckovic N, Capo I, Levakov AF. Congenital cytomegalic inclusion disease with disseminated Herpes simplex infection. Malays J Pathol. 2019; 41(1):75-78.
22. Kiyokoba R, et al. Fetal cytomegalovirus infection manifesting as transient pancytopenia. Congenit Anom (Kyoto). 2015; 55(3):164-6. doi:10.1111/cga.12104.
23. Leruez-Ville M, Ville Y. Cytomegalovirus infection in pregnancy. Presse Med. 2014; 43(6 Pt 1):683-90. doi:10.1016/j.lpm.2014.02.016.
24. Lazzarotto T, Guerra B, Gabrielli L, Lanari M, Landini MP. Update on the prevention, diagnosis and management of cytomegalovirus infection during pregnancy. Clin Microbiol Infect. 2011; 17(9):1285-93. doi: 10.1111/j.1469-0691.2011.03564.x.
25. Leruez-Ville M, Ville Y. Fetal cytomegalovirus infection. Best Pract Res Clin Obstet Gynaecol. 2017; 38:97-107. doi: 10.1016/j.bpobgyn.2016.10.005.
26. Leruez-Ville M, et al. In utero treatment of congenital cytomegalovirus infection with valacyclovir in a multicenter, open-label, phase II study. Am J Obstet Gynecol. 2016; 215(4):462.e1-462.e10. doi: 10.1016/j.ajog.2016.04.003.
27. Koelle DM, et al. Worldwide circulation of HSV-2×HSV-1 recombinant strains. Sci Rep. 2017; 7:44084. doi:10.1038/srep44084.
28. Johnston C, Corey L. Current Concepts for Genital Herpes Simplex Virus Infection: Diagnostics and Pathogenesis of Genital Tract Shedding. Clin Microbiol Rev. 2016; 29(1):149-61. doi:10.1128/CMR.00043-15..
29. Monroe-Wise A, et al. Genital Ulcer Disease. The Travel and Tropical Medicine Manual (Fifth Edition). Elsevier. 2017.
30. Johnston C, Wald A. Genital Herpes Infectious Diseases (Fourth Edition). Elsevier. 2017; Vol. 1, pp. 567-574.e2.
31. Johnston C, Wald A. Genital Herpes. Atlas of Sexually Transmitted Diseases and AIDS (Fourth Edition). Elsevier. 2010.
32. Hay P, Pittrof R. Infections in pregnancy. Antibiotic and Chemotherapy (Ninth Edition). Elsevier. 2010.
33. Zoltan E. Sexually Transmitted Disease. Penn Clinical Manual of Urology. Elsevier. 2007.
34. Stephenson-Famy A, Gardella C. Herpes simplex virus infection during pregnancy. Obstet Gynecol Clin North Am. 2014; 41(4):601-14, doi: 10.1016/j.ogc.2014.08.006.
35. Lee R, Nair M. Diagnosis and treatment of herpes simplex 1 virus infection in pregnancy. Obstet Med. 2017; 10(2):58-60, doi: 10.1177/1753495X 16689434.
36. Dineen R, Jaspan T. The neonatal brain. Clinical Ultrasound (Third Edition). Elsevier. 2011.
Articole din ediţiile anterioare
Valoarea predictivă a indicilor Doppler ai arterei uterine la 11-14 săptămâni pentru complicaţiile hipertensive ale sarcinii
Introducere. Complicaţiile hipertensive ale sarcinii pot duce adesea la situaţii grave, chiar cu potenţial letal pentru mamă şi făt. Ecografia Dopp...
Rezultatele sarcinii la femeile cu artrită psoriazică
Psoriazisul este o boală inflamatorie cronică, având consecinţe fizice şi psihologice importante. Acesta se caracterizează printr-un proces inflama...
Profilul de risc clinic asociat cancerului ovarian
Acest studiu a fost efectuat pentru a evalua caracteristicile profilului de risc clinic al pacientelor cu tumori ovariene care au fost tratate chir...