Noi tratamente sistemice în infecţia cu HPV
New systemic treatments in HPV infection
Abstract
Human infection with various genotypes of human papillomaviruses (HPV) is one of the most common sexually transmissible viral infections. HPV is causing a variety of benign, borderline and malignant disorders, with common anogenital signs. The therapy of cutaneous and mucosal infections with confirmed HPV involves local and/or systemic treatment, applied separately or in combination. HPV requires antiviral systemic treatment, but at present there isn’t an antiviral systemic medication approved against HPV infections. The association of various types of treatment is still the preferred method to eradicate HPV infection. This paper offers information about possible systemic treatments of HPV infection, based on the documentation from the PubMed database (1989-2018), including immunomodulatory drugs, antiviral medications, therapeutic HPV vaccines and biological therapy. More studies are still needed with similar models, with multiple biological samples and with follow-up for periods long enough to allow a comparison of the short-term/long-term effectiveness of various types of systemic treatment for HPV.Keywords
HPVHPV systemic treatmenttherapeutic vaccinessystemic immunomodulatorssystemic antiviral drugsRezumat
Infecţia umană cu diferite genotipuri ale virusului papiloma uman (HPV) este una dintre cele mai frecvente infecţii virale cu transmitere sexuală. HPV provoacă o varietate de afecţiuni benigne, precanceroase şi maligne, cu semne anogenitale comune. Terapia infecţiilor cutanate şi mucoase cu HPV confirmate implică tratament local şi/sau sistemic, aplicat separat sau în combinaţie. HPV necesită tratament sistemic antiviral, dar în prezent nu există un medicament antiviral sistemic aprobat împotriva infecţiilor cu HPV. Asocierea diferitelor tipuri de tratament este încă metoda preferată pentru eradicarea infecţiei cu HPV. Această lucrare oferă detalii despre posibilele tratamente sistemice ale infecţiei cu HPV, pe baza documentaţiei din baza de date PubMed (1989-2018), inclusiv medicamente imunomodulatoare, medicamente antivirale, vaccinuri HPV terapeutice şi terapie biologică. Sunt necesare mai multe studii cu modele similare, cu multiple probe biologice şi cu perioade de urmărire suficient de lungi pentru a permite o comparaţie a eficacităţii pe termen scurt/lung a diferitelor tipuri de tratament sistemic în infecţia dovedită cu HPV.Cuvinte Cheie
HPVtratament sistemic HPVvaccinuri terapeuticeimunomodulatoare sistemicemedicamente antivirale sistemiceGenital infections with human papillomaviruses (HPV), among the most common sexually transmitted diseases (STD), generate alarming signals in human health, due to the prevalence and dissemination, as well as to various pathologies induced mostly at the level of the genital tract(1). The genomic analysis of the HPV viruses, ADNds viruses, describes ~210 HPV genotypes (IHPRC), over 40 genotypes, with high risk (hr) and low risk (lr), including 12 HPVshr/IARC which can infect mucous membranes and the skin, causing benign, borderline and malignant lesions(2). The virus has tropism for feminine genital tract. Among adults aged 18-59 years old (CDC: USA, 2013-2014), 45% of men and approximately 40% of women had genital HPV infection, and approximately 25% of men and 20% of women had genital infection with HPVshr. The prevalence of the oral infection with HPV in adults aged 18-69 years old (CDC: USA, 2011-2014) was approximately 7%, HPVshr being present in 4% of cases(3).
The infection with various genotypes of HPV causes skin warts, genital warts, papillomas (anogenital, oropharyngeal/laryngeal), hyperkeratosis, precursor lesions and various cancers (uterine and other genital cancers, orogenital, laryngeal/pharyngeal, non-melanoma skin cancer)(4).
Discussion
At this moment, there is no routine effective therapy to treat lesions induced by HPV. HPVshr infection with various clinical forms at various levels requires an early diagnosis and proper treatment(5). The treatment improves symptoms, especially regarding evolutive flares, lowering the possibility of infection by HPV and possibly through other possible STD associated(6). It is recommended that all clinical and subclinical susceptible lesions at the level of epithelium and mucous membranes be tested for HPV(7). The possibility of identifying the genotype is useful, especially for infections with HPVshr, when the therapy must be prompt and targeted, and post-therapeutic follow-up must be done for long term in order to prevent the neoplastic transformation(8). Taking into account the fact that many lesions with confirmed HPV etiology have a self-limiting evolution, it is recommended that before starting the treatment the patient should be kept under observation for several weeks, expecting the spontaneous healing(9).
A multidisciplinary approach is required for optimal decision making, treatment planning, and post-treatment response assessment. The therapy of the infections produced by HPV is local and systemic. Topical treatment removes lesions, but the absence of infection is not guaranteed(10). Untreated, some injuries may regress spontaneously, others grow in size and number, and they may evolve as benign/malignant(11). The absence of a sterilizing therapy diminishes the effectiveness of evaluation and monitorization of the sexual partners, but their testing can identify ignored lesions(12).
The treatment of cutaneous and mucous infections involves topical and/or systemic treatment applied separately or combined(13). The local treatments include therapeutic chemical agents (cytotoxic agents: trichloroacetic acid 80-90%, podophyllin, podophyllotoxin, 5-fluorouracil, imiquimod 5% cream, retinoids, topical interferon, intralesional interferon) and/or physical therapeutic agents (ablative therapy: cryotherapy, CO2 laser, LEEP – loop resection), or surgical excision(14,15). HPV is ubiquitous, especially in the lower genital tract, which makes the pharmacological treatment to be considered as the therapy of choice(16). The treatment with interferon and immunomodulating agents (inosine) did not bring the presumed results (many side effects which limited the therapeutic doses)(17).
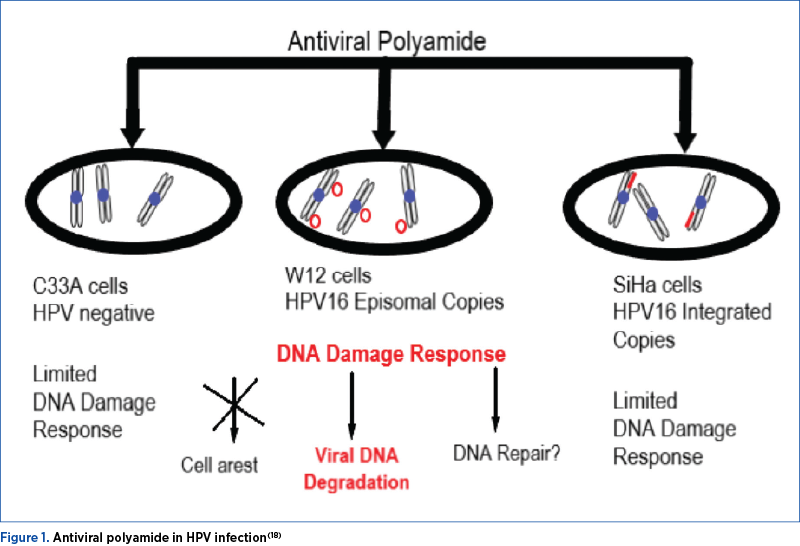
Systemic antiviral treatment would be the treatment of choice, but at present there is no systemic antiviral drug approved for HPV(19). Combining multiple types of treatments, correlated with the appearance of lesions and/or with the presence of asymptomatic HPV, is still commonly used in the idea of therapeutic success. This article details the progresses in the treatment of systemic infection with HPV, regardless of type, location and genotype(20).

This paper provides up-to-date scientific data on the possible systemic treatments of human infections with HPV confirmed etiology. Documentation, made during the period July-December 2018 on PubMed/Medline database, included the study of essays and original articles between 1997 and 2017. The study used the following keywords: HPV, HPV systemic treatments, prophylactic anti-HPV vaccines, therapeutic HPV vaccines, antiviral systemic medications, systemic immunomodulators.
Among the options available currently, only surgical treatment has a primary clearance rate approaching 100%. Recurrences occur after all therapies. Recurrence rates, including new lesions at previously treated or new sites, are often as high as 20-30%, and increase with longer duration of the follow-up period. All topical treatments are associated with local skin reactions including itching, burning, erosions and pain. A number of studies highlighted the role of HPV systemic treatment, from which the most prevalent are aromatic retinoids, interferon, inosine, while other studies have reported benefits after using hydroxicloroquine(22).
Antiviral medications like foscarnet, cidofovir and ribavirin were used as systemic HPV treatment with good results(23).
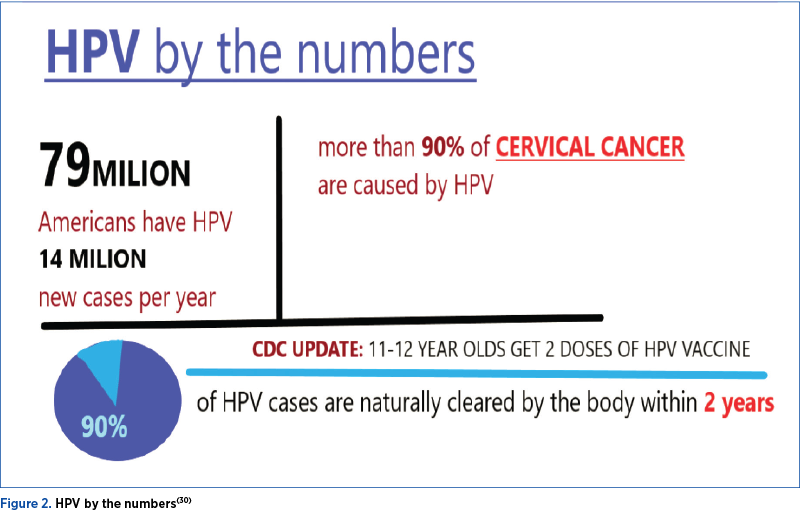
A history of genital warts, a positive HPV test result, or abnormal cervical, vaginal, vulvar or anal cytology, all indicate a prior HPV infection, but not necessarily with the HPV types included in the vaccines. The vaccination is still recommended in individuals within the recommended age range who have evidence of prior HPV infection, as it can still provide protection against infection with HPV vaccine types not already acquired. However, these patients should be advised that vaccination will have no therapeutic effect on preexisting HPV infection or in HPV-associated diseases, and the potential benefit of HPV vaccination is not as great as if they were vaccinated before their sexual debut(24,25).
The therapeutic anti-HPV vaccines are supposed to induce cell-mediated immunity and to prevent the development of benign and malignant lesions induced by this virus. Cell-mediated rather than humoral immune responses are important for the clearance of established infections. It has been observed that spontaneous clearance and slow progression of HPV infections are associated with a strong cell-mediated immune response involving mainly T-helper type 1 cells and cytotoxic T-cells derived from CD4+ and CD8+ T-cells, respectively. The HPV E6 and E7 oncoproteins are essential for the onset and maintenance of malignancy; thus, they are unlikely to escape immune responses by mutation. They are also expressed constitutively and at high levels, and therefore represent near-ideal targets for the development of therapeutic vaccines against established HPV infections and lesions. Other proteins useful for targeting of early viral infections are E1 (viral helicase) and E2, which are expressed at higher levels than E6 and E7 at very early stages before viral genome integration.
An ideal therapeutic vaccine would target these proteins to induce strong tumor-specific T-cell type 1 and cytotoxic lymphocyte (CTL) responses able to kill infected and malignant cells(26). The types of therapeutic vaccines, which are developed and evaluated at the moment, are vaccines with viral or bacterial vectors, vaccines based on dendritic cells and modified tumor cells, vaccines based on peptides, proteins or RNA replicons or DNA vaccines(27,28). Although some clinical trials testing the efficiency of the therapeutic HPV vaccines are being under assessment, there isn’t yet a commercial therapeutic vaccine available(1,29).
Progress will likely come from clinical trials testing the treatment of low-grade lesions of the cervix, with the aim of accelerated and sustained resolution following either HPV immune response, or drug treatments as the stepping stone for wider therapeutic application(31).
The treatment is reserved for patients with visible warts. The general treatment strategy is to eliminate as many of the visible lesions as possible until the host immune system can control the viral replication(32). The treatment is not recommended for subclinical anogenital or mucosal human papillomavirus infection in the absence of coexistent dysplasia(33).
The current strategies in systemic treatment against HPV tend to focus on inhibiting the replication of viral DNA and on modulation of the body’s immune response(34,35). Recent research directions are promising to provide antiviral medications anti-HPV with the characteristics aforementioned(36,37). However, there is still need of large population clinical trials, with diversified biological samples and with long enough follow-up periods to allow comparison of the short-term/long-term efficacy of the different types of systemic anti-HPV treatments(38,39).
However, current therapeutic strategies remain expensive due to the systems of manufacture, meaning that even if a vaccine were to be commercialized, its cost would render it less accessible to populations in developing countries, where the burden of cervical cancer is highest. To expand the impact of therapeutic vaccines in developing countries, plant-made products are a promising technology to offer inexpensive and effective pharmaceuticals(39). Additionally, cold-chain vaccine delivery is necessary to maintain vaccine shelf life, and contributes to the inaccessibility of vaccine products in developing countries due to poor infrastructure(40).
Conclusions
Even though the importance of this viral infection is well known worldwide and many studies were conducted on this topic, the ideal systemic treatment for infections determined by HPV isn’t yet close to being discovered.
Vaccination at a very large scale prior the sex life debut is for now the only tool we have in the battle against HPV infections. Still, as several candidate vaccines are tested nowdays in clinical trials, it is likely that we will benefit from a therapeutic HPV vaccine in the near future.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
1. Lahiri CD, Dugan KB, Xie X, Reimers L, Burk RD, et al. Oral Lopinavir Use and Human Papillomavirus Infection in HIV-positive Women. J Acquir Immune Defic Syndr. 2015; 70(2):e63-6.
2. Yanofsky VR, Patel RV, Goldenberg G. Genital warts: a comprehensive review. J Clin Aesthet Dermatol. 2012; 5(6):25-36.
3. Kelly H, Weiss HA, Benavente Y, de Sanjose S, Mayaud P; ART and HPV Review Group. Association of antiretroviral therapy with high-risk human papillomavirus, cervical intraepithelial neoplasia, and invasive cervical cancer in women living with HIV: a systematic review and meta-analysis. Lancet HIV. 2018; 5(1):e45-e58.
4. Bitsadze VO, Khamani NM, Makatsariya NA. Role of inosine pranobex in management of HPV-associated diseases: problems and prospective. Obstetrics, Gynecology and Reproduction. 2016; 10(3): 76-84.
5. Olsen EA, Kelly FF, Vollmer RT, Buddin DA, Weck PK. Comparative study of systemic interferon alfa-NL and isotretinoin in the treatment of resistant condylomata acuminata. J Am Acad Dermatol. 1989; 20(6):1023-30.
6. Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015; 64(RR-03):1-137.
7. Mariani L, Vici P, Suligoi B, Checcucci-Lisi G, Drury R. Early Direct and Indirect Impact of Quadrivalent HPV (4HPV) Vaccine on Genital Warts: a Systematic Review. Adv Ther. 2015; 32:10-30.
8. Hampson L, Martin-Hirsch P, Hampson IN. An overview of early investigational drugs for the treatment of human papilloma virus infection and associated dysplasia. Expert Opin Investig Drugs. 2015; 24(12):1529-1537.
9. Yliskoski M, Syrjänen K, Syrjänen S, Saarikoski S, Nethersell A. Systemic α-interferon (Wellferon) treatment of genital human papillomavirus (HPV) type 6, 11, 16, and 18 infections: double-blind, placebo-controlled trial. Gynecol Oncol. 1991; 43(1): 55-60.
10. Mlynarczyk-Bonikowska B, Majewska A, Malejczyk M, Mlynarczyk G, Majewski S. Antiviral medication in sexually transmitted diseases. Part I: HSV, HPV. Mini Rev Med Chem. 2013; 13(13):1837-45.
11. Butureanu S, Butureanu TA. Human Papilloma Virus Infection and Cervical Carcinogenesis. Obstetrica şi Ginecologia. 2016; 64(2):69-73.
12. Heard I, Palefsky JM, Kazatchkine MD. The impact of HIV antiviral therapy on human papillomavirus (HPV) infections and HPV-related diseases. Antivir Ther. 2004; 9(1):13-22.
13. Moscicki A-B, Palefsky JM. HPV in men: an update. J Low Genit Tract Dis. 2011; 15(3):231-234.
14. Petca A, Oprescu C, Radu D, Petca R, Burnei-Russu A, Strajean D, Zvâncă M, Boţ M. Management of cervical dysplasia in the context of pregnancy. Ginecologia.ro. 2017; 5(17):14-18.
15. Goidescu I, Rotar I, Stamatian F, Mureşan D. Cervical cancer during pregnancy. Diagnostic and treatment options. Obstetrica şi Ginecologia. 2016; 64(1):1-8.
16. Sinha S, Relhan V, Garg VK. Immunomodulators in warts: Unexplored or ineffective?. Indian J Dermatol. 2015; 60(2):118-129.
17. Batman G, Oliver AW, Zehbe I, Richard C, Hampson L, et al. Lopinavir up-regulates expression of the antiviral protein ribonuclease L in human papillomavirus-positive cervical carcinoma cells. Antivir Ther. 2011; 16(4):515-25.
18. https://www.semanticscholar.org/paper/Recent-Insights-into-the-Control-of-Human-(HPV)-and-Fisher/
19. Tsambaos D, Georgiou S, Monastirli A, Sakkis T, Sagriotis A, et al. Treatment of condylomata acuminata with oral isotretinoin. J Urol. 1997; 158(5):1810-2.
20. Georgala S, Katoulis AC, Georgala C, Bozi E, Mortakis A. Oral isotretinoin in the treatment of recalcitrant condylomata acuminata of the cervix: a randomised placebo controlled trial. Sex Transm Infect. 2004; 80:216-8.
21. https://npwomenshealthcare.com/boosting-hpv-vaccination-rates-call-action/
22. Van Valckenborgh I, Wellens W, De Boeck K, Snoeck R, De Clercq E, et al. Systemic Cidofovir in Papillomatosis. Clin Infect Dis. 2001; 32(3):e62-e64.
23. Polansky H, Itzkovitz E, Javaherian A. Human papillomavirus (HPV): systemic treatment with Gene-Eden-VIR/Novirin safely and effectively clears virus. Drug Des Devel Ther. 2017; 11:575-83.
24. Kim DK, Hunter P, Advisory Committee on Immunization Practices. Recommended Adult Immunization Schedule, United States, 2019. Ann Intern Med. 2019; 170(3):182-92.
25. Markowitz LE, Dunne EF, Saraiya M, Chesson HW, Curtis CR, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2014; 63(RR-05):1-30.
26. Yang A, Farmer E, Wu TC, Hung CF. Perspectives for therapeutic HPV vaccine development. J Biomed Sci. 2016; 23:75.
27. Hancock G, Hellner K, Dorrell L Therapeutic HPV vaccines. Best Pract Res Clin Obstet Gynaecol. 2018; 47:59-72.
28. Braaten KP, Laufer MR. Human Papillomavirus (HPV), HPV-Related Disease, and the HPV Vaccine. Rev Obstet Gynecol. 2008; 1(1):2-10.
29. Menon S, Rossi R, Zdraveska N, Kariisa M, Acharya SD, et al. Associations between highly active antiretroviral therapy and the presence of HPV, premalignant and malignant cervical lesions in sub-Saharan Africa, a systematic review: current evidence and directions for future research. BMJ Open. 2017; 7(8):e015123.
30. https://www.siumed.edu/cancer/highlights/hpv-vaccine-safe-my-child.html
31. Lacey CJN. Therapy for genital human papillomavirus-related disease. J Clin Virol. 2005; 32(suppl):S82-S90.
32. Popescu SD, Bănică AM, Vlădăreanu S, Vlădăreanu R. Human Papilloma Virus – neonatal involvement. Ginecologia.ro. 2018; 6(20)60-63.
33. Pavan MHP, Velho PENF, Vigani AG, Gonçalves Jr FL, Aoki FH. Treatment of human papillomavirus with peg-interferon alfa-2b and ribavirin. Braz J Infect Dis. 2007; 11(3):383-4.
34. Gerein V, Rastorguev E, Gerein J, Jecker P, Pfister H. Use of interferon-alpha in recurrent respiratory papillomatosis: 20-year follow-up. Ann Otol Rhinl Laryngol. 2005; 114(6):463-71.
35. Purceli MCSC, Jotz GP, Miranda SL, et al. The use of ribavirin in the treatment of recurrent respiratory papillomatosis (PRR). Acta AWHO. 1996; 15(1):33-6.
36. De Clercq E. Clinical Potential of the Acyclic Nucleoside Phosphonates Cidofovir, Adefovir, and Tenofovir in Treatment of DNA Virus and Retrovirus Infections. Clin Microbiol Rev. 2003; 16(4):569-96.
37. Wagstaff AJ, Bryson HM. Foscarnet. A reappraisal of its antiviral activity, pharmacokinetic properties and therapeutic use in immunocompromised patients with viral infections. Drugs. 1994; 48(2):199-226.
38. Coremans G, Snoeck R. Cidofovir: clinical experience and future perspectives on an acyclic nucleoside phosphonate analog of cytosine in the treatment of refractory and premalignant HPV-associated anal lesions. Expert Opin Pharmacother. 2009; 10(8):1343-52.
39. Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012; 30(Suppl 5):F12-F23.
40. Hefferon K. Plant virus expression vectors: a powerhouse for global health. Biomedicines. 2017; 5(3):E44.





