Uterine adenomyosis, characterized by the ectopic presence of endometrial tissue in the myometrium, has a significant impact on the quality of life of reproductive aged women. Therefore, its prompt diagnosis is essential for symptom relief, but it is often established histologically, after hysterectomy. Noninvasive diagnostic methods have started to gain ground recently, but research is still ongoing. Transvaginal ultrasound (TVUS) has proven itself an essential diagnostic tool, but is less accurate than magnetic resonance imaging in differentiating adenomyosis from uterine leiomyoma. In order to increase the accuracy of the method, the combined use of TVUS and elastography has been attempted, with strain elastography showing promising results. Biomarkers such as interleukin 6, tumour antigens, circulating endometrial cells or microRNAs could represent novel diagnostic tools, but still require further validation in larger-based population studies. Conclusions. The diagnosis of adenomyosis is still challenging, with novel imaging and serological methods emerging as future potential noninvasive assets. Further research is necessary to warrant their diagnostic utility, as well as their capacity of distinguishing adenomyosis from other benign uterine pathologies.
Provocări în diagnosticul neinvaziv al adenomiozei
Challenges in the noninvasive diagnosis of adenomyosis
First published: 23 octombrie 2020
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/ObsGin.68.3.2020.4011
Abstract
Rezumat
Introducere. Adenomioza, definită ca prezenţa ectopică a ţesutului endometrial la nivelul miometrului, are un impact semnificativ asupra calităţii vieţii femeilor de vârstă reproductivă. Prin urmare, diagnosticul prompt al acesteia este esenţial pentru ameliorarea simptomatologiei, dar este deseori stabilit ca urmare a histerectomiei, prin intermediul examenului histopatologic. Metodele de diagnostic neinvaziv au început să câştige teren în ultimul timp, dar cercetarea pe această temă este încă în curs de desfăşurare. Ecografia transvaginală (ETV) s-a dovedit a fi un instrument de diagnostic esenţial, dar este mai puţin precisă decât rezonanţa magnetică nucleară în diferenţierea adenomiozei de leiomiomatoza uterină. Pentru a creşte acurateţea metodei, a fost încercată utilizarea combinată a ETV şi a elastografiei, elastografia calitativă demonstrând rezultate promiţătoare. Biomarkeri precum interleukina 6, antigenii tumorali, celulele endometriale circulante sau microARN-urile ar putea constitui noi tehnici de diagnostic, dar necesită validare în studii pe populaţii mai mari. Concluzii. Diagnosticul adenomiozei constituie în continuare o provocare, metodele inovatoare imagistice şi serologice putând constitui viitoare noi tehnici diagnostice neinvazive. Studii viitoare sunt necesare pentru a confirma utilitatea lor, precum şi pentru a stabili capacitatea acestora de a distinge adenomioza de alte patologii uterine benigne.
Introduction
Endometriosis is characterized by chronic inflammation and the presence of functional endometrial gland and stroma outside the uterine cavity(1).
Primary endometriosis foci may be present in the pelvic compartment and ovaries, their growth being dependent on oestrogen secretion(2,3). Therefore, this condition affects women of reproductive age, causing chronic pelvic pain, dyspareunia and infertility(2). Symptoms subside and progressively disappear with the onset of menopause, but oestrogen-dependent proliferation produces the invasion of healthy tissue, in a malignant fashion(4).
Research on adenomyosis has been limited in the past, with more detailed data on the subject being published in the past two decades(5). Uterine adenomyosis is defined by the presence of endometrial gland in the myometrium, accompanied by hypertrophy or hyperplasia of the surrounding myometrium. Its clinical manifestations are similar to those of other types of endometriosis, but menorrhagia and dysmenorrhea are more frequently encountered(6). Hence, various imagistic techniques have been proposed for an accurate and rapid diagnosis, but a lack of standardised ultrasonographical features makes it difficult to assess the exact sensitivity and specificity of magnetic resonance imaging (MRI) and transvaginal ultrasound (TVUS)(7,8).
Different classification systems have been proposed, based on the extent/severity of endometriosis. The most widely used is the revised American Society for Reproductive Medicine score (stage I: minimal; stage II: mild; stage III: moderate; stage IV: severe)(9). However, the stage of the disease does not correlate with the severity of symptoms, the frequently delayed diagnosis being a major concern with this condition. The gold standard in the diagnosis of endometriosis today still remains laparoscopy, accompanied by inspection of peritoneal cavity and microscopic examination of incriminating lesions(9). In an effort to facilitate the early recognition of endometriosis, current studies are investigating various noninvasive diagnostic methods, such as imagistic tools, inflammatory biomarkers, genetic tests or microRNAs(5,10).
Both endometriosis and adenomyosis require a prompt diagnosis, due to the impact they pose upon the health-related quality of life of young women. The aim of this paper is to emphasize the need for development of novel, noninvasive diagnostic strategies of adenomyosis, in light of recent literature data.
Transvaginal ultrasound versus MRI
TVUS has emerged as an useful tool in the diagnosis of deep, infiltrating endometriosis, providing a reliable preoperative diagnosis for the surgeon, without the need of performing an MRI(11). Nevertheless, TVUS poses advantages over MRI, as it is cheaper, easily available and more familiar to the gynaecologist. The accuracy of these imaging techniques is similar, but TVUS is, unfortunately, less sensitive than MRI in the case of concomitant leiomyoma, according to a review published by Hoyos et al.(12)
Cunningham et al. described certain typical 2D sonographic signs, suggestive of adenomyosis: globular uterine enlargement, heterogenous areas within the myometrium, asymmetry of the uterine walls, hypoechoic striations in the myometrium, anechoic lacunae/myometrial cysts(13). These sonographic diagnostic criteria were described and unanimously accepted after the meeting of the Morphological Uterus Sonographic Assessment Group (MUSA)(14). Out of these features, asymmetry of the uterine walls and anechoic lacunae/myometrial cysts proved to be the most frequently associated with adenomyosis(15). Moreover, various authors suggest that the diagnosis should be based upon at least two MUSA criteria(15,16).
Van den Bosch et al. reviewed the literature data regarding the diagnostic accuracy of ultrasound in adenomyosis. They concluded that the aforementioned MUSA criteria are not patognomonic for adenomyosis, as myometrial cysts/anechoic lacunae and hypoechoic striations in the myometrium can raise serious differential diagnostic problems with uterine fibromatous nodules(16). These nodules have a very variable sonographic aspect. They are typically described as well-defined hypo-, iso- or hyperechogenic structures, but they can have an irregular aspect and inhomogeneous echogenicity due to calcareous degeneration. In such situations, or in cases in which adenomyosis and uterine leiomyoma coexist, the differential diagnosis can be challenging and an MRI is recommended for elucidating the pathological findings(12,17).
TVUS combined with elastography
Although TVUS still remains an important asset, the use of 2D ultrasonography in combination with elastography has been believed to improve the diagnostic accuracy of adenomyosis. There are two types of elastography, shear wave (quantitative) and strain (qualitative). Both of them have been studied for the diagnosis of adenomyosis.
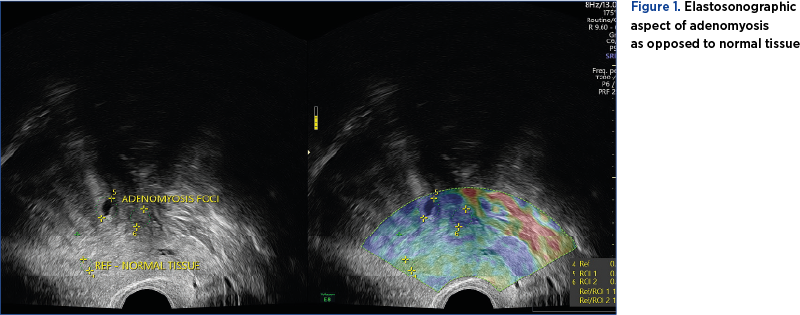
Shear wave elastography can identify lesion stiffness, expressed through an absolute value in kPa. Strain, qualitative elastography can provide information regarding the relative tissue stiffness by comparing presumptive healthy tissue with presumptive pathological tissue (Figure 1)(18).
Zhang et al. conducted a prospective study on 34 premenopausal patients, with uterine benign pathology and presenting with pelviabdominal pain and/or metrorrhagia. The quantitative elastography proved to be efficient in detecting adenomyosis foci, by detecting an increase in stiffness tissue as opposed to the healthy surrounding myometrial tissue. However, it failed to differentiate atypical uterine leiomyoma from adenomyosis if the two entities coexisted(19).
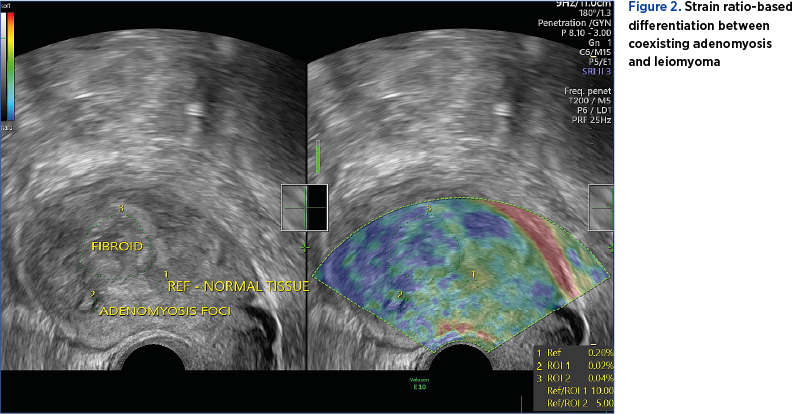
In contrast with the aforementioned study, Liu et al. support the use of qualitative elastography in diagnosing adenomyosis, claiming that its accuracy is similar to the one of MRI(20). Furthermore, the study proves that TVUS combined with elastography can differentiate uterine leiomyoma from adenomyosis with an accuracy similar to the one of MRI based on the numeric value of strain ratio (Figure 2)(20).
Both techniques require validation in future studies, with strain elastography showing more promising results. The experience of the examiner and the technical equipment also play an important role, both for TVUS and elastography(16).
Biomarkers
The physiopathology and etiology of endometriosis are not yet fully understood. Therefore, various recent studies have tried to elucidate the involvement of various biomarkers in the pathogenesis of this condition. Cytokines, markers of apoptosis, chemotactic molecules, as well as several genes have all proven to play essential roles in the pathways of abnormal implantation of endometrial tissue(21). Among these, interleukin-6 (IL-6), cancer antigen 125 (CA-125) and cancer antigen 199 (CA-199) have been intensly studied as potential biomarkers of endometriosis(22). IL-6, in spite of its involvement in the implantation of endometrial foci, does not increase significantly in patients with endometriosis as opposed to healthy counterparts(23,24).
A meta-analysis performed on small scale studies suggested that serum CA-125 can be used as a reliable, single diagnostic marker in women presenting with symptoms of endometriosis(25). However, other studies underlined its importance only in patients with advanced types of endometriosis(26), and the fluctuation of its levels in relation to phases of the menstrual cycle(27). Similar findings were reported in the case of CA-199, which is also influenced by hormonal secretion and failed to prove itself as a meaningful diagnostic test, when used alone(28).
Circulating endometrium cells (CECs) have benefited from a lot of attention recently, a Chinese study proving that these are detectable in the blood of patients with endometriosis. Their presence highlights the capacity of endometrial tissue to spread through peripheral blood, especially in the case of intrapelvic endometriosis. However, as this study involved a small number of subjects and an even smaller number of patients diagnosed with adenomyosis, further studies on larger populations are necessary for validation(29).
Therefore, as studies of currently trending noninvasive biomarkers have failed to obtain reliable results, the possibility of using these molecules as diagnostic tools for endometriosis is still questionable(22).
MicroRNAs
Ectopic endometrium tissue leads to a chronic inflammatory status, which can be responsible for genic transcription abnormalities and dysregulation of post-translational mechanisms. MicroRNAs, small fragments of non-coding DNA, control the genetic expression through translation inhibition or messenger RNA degradation(30). Therefore, they play an important role in the epigenetic mechanisms responsible for the progesterone-resistant and estrogen-susceptible eutopic endometrial tissue in patients with endometriosis(30). A Chinese study performed on a small-sample size population demonstrated that plasma miR-17-5p, miR-20a and miR-22 are downregulated in patients with stage III and IV endometriosis(31). Another study found an important expression dysregulation of miR-9 and miR-34 miRNA in the eutopic secretory endometrium of women with endometriosis(32). Downregulation of let-7b and miR-135a has also been reported in conjunction with endometriosis, Cho et al. suggesting that these microRNAs have the potential of becoming future noninvasive diagnostic biomarkers. However, the same study recommends the simultaneous use of multiple microRNAs for diagnostic purposes, as well as the necessity of comparing seric and tissular markers of the same type in further studies(33). A Cochrane systematic review recommends the need for further validation of role of microRNAs in further studies as well, highlighting the lack of standardized methods for the detection of various types of these non-coding DNAs, which impacts research reproducibility(28).
Conclusions
The diagnosis of adenomyosis is still challenging, with novel imaging and serological methods emerging as future potential noninvasive assets. The addition of strain elastography seemed to increase the diagnostic accuracy of TVUS, the combined method also being able to distinguish adenomyosis from uterine leiomyoma. Noninvasive biomarkers and microRNAs have showed several intriguing results, but the available data are only based upon small scale population studies. Therefore, further, wider-based population research is necessary to warrant the diagnostic utility of currently studied noninvasive imaging and serological techniques.
Bibliografie
-
1. Greene AD, Lang SA, Kendziorski JA, Sroga-Rios JM, Herzog TJ, Burns KA. Endometriosis: Where are We and Where are We Going? Reprod Camb Engl. 2016 Sep;152(3):R63–78.
-
2. Bulun SE. Endometriosis. N Engl J Med. 2009 Jan 15;360(3):268–79.
-
3. Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014 May;10(5):261–75.
-
4. Flores I, Rivera E, Ruiz LA, Santiago OI, Vernon MW, Appleyard CB. Molecular profiling of experimental endometriosis identified gene expression patterns in common with human disease [Internet]. Fertil Steril. 2007 May;87(5):1180–99.
-
5. Benagiano G, Brosens I, Habiba M. Adenomyosis: a life-cycle approach. Reprod Biomed Online. 2015 Mar 1;30(3):220–32.
-
6. Protopapas A, Grimbizis G, Athanasiou S, Loutradis D. Adenomyosis: Disease, uterine aging process leading to symptoms, or both? Facts Views Vis ObGyn. 2020 Aug 5;12(2):91–104.
-
7. Tellum T, Matic GV, Dormagen JB, Nygaard S, Viktil E, Qvigstad E, et al. Diagnosing adenomyosis with MRI: a prospective study revisiting the junctional zone thickness cutoff of 12 mm as a diagnostic marker. Eur Radiol. 2019 Dec;29(12):6971–81.
-
8. Andres MP, Borrelli GM, Ribeiro J, Baracat EC, Abrão MS, Kho RM. Transvaginal Ultrasound for the Diagnosis of Adenomyosis: Systematic Review and Meta-Analysis. J Minim Invasive Gynecol. 2018 Feb 1;25(2):257–64.
-
9. Kiesel L, Sourouni M. Diagnosis of endometriosis in the 21st century. Climacteric J Int Menopause Soc. 2019;22(3):296–302.
-
10. Agrawal S, Tapmeier T, Rahmioglu N, Kirtley S, Zondervan K, Becker C. The miRNA Mirage: How Close Are We to Finding a Non-Invasive Diagnostic Biomarker in Endometriosis? A Systematic Review. Int J Mol Sci. 2018 Feb 17;19(2):599.
-
11. Turocy JM, Benacerraf BR. Transvaginal sonography in the diagnosis of deep infiltrating endometriosis: A review. J Clin Ultrasound. 2017 Jul 8;45(6):313–8.
-
12. Hoyos LR, Benacerraf B, Puscheck EE. Imaging in Endometriosis and Adenomyosis. Clin Obstet Gynecol. 2017;60(1):27–37.
-
13. Cunningham RK, Horrow MM, Smith RJ, Springer J. Adenomyosis: A Sonographic Diagnosis. RadioGraphics. 2018 Sep 1;38(5):1576–89.
-
14. Van den Bosch T, Dueholm M, Leone FPG, Valentin L, Rasmussen CK, Votino A, et al. Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: a consensus opinion from the Morphological Uterus Sonographic Assessment (MUSA) group. Ultrasound Obstet Gynecol. 2015;46(3):284–98.
-
15. Pinzauti S, Lazzeri L, Tosti C, Centini G, Orlandini C, Luisi S, et al. Transvaginal sonographic features of diffuse adenomyosis in 18-30-year-old nulligravid women without endometriosis: association with symptoms. Ultrasound Obstet Gynecol. 2015;46(6):730–6.
-
16. Van den Bosch T, Van Schoubroeck D. Ultrasound diagnosis of endometriosis and adenomyosis: State of the art. Best Pract Res Clin Obstet Gynaecol. 2018 Aug 1;51:16–24.
-
17. Guerriero S, Saba L, Pascual MA, Ajossa S, Rodriguez I, Mais V, et al. Transvaginal ultrasound vs. magnetic resonance imaging for diagnosing deep infiltrating endometriosis: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2018;51(5):586–95.
-
18. Dietrich CF, Barr RG, Farrokh A, Dighe M, Hocke M, Jenssen C, et al. Strain Elastography – How To Do It? Ultrasound Int Open. 2017 Sep;3(4):E137–49.
-
19. Zhang M, Wasnik AP, Masch WR, Rubin JM, Carlos RC, Quint EH, et al. Transvaginal Ultrasound Shear Wave Elastography for the Evaluation of Benign Uterine Pathologies: A Prospective Pilot Study. J Ultrasound Med Off J Am Inst Ultrasound Med. 2019 Jan;38(1):149–55.
-
20. Liu X, Ding D, Ren Y, Guo S-W. Transvaginal Elastosonography as an Imaging Technique for Diagnosing Adenomyosis. Reprod Sci Thousand Oaks Calif. 2018;25(4):498–514.
-
21. Laganà AS, Vitale SG, Salmeri FM, Triolo O, Ban Frangež H, Vrtačnik-Bokal E, et al. Unus pro omnibus, omnes pro uno: A novel, evidence-based, unifying theory for the pathogenesis of endometriosis. Med Hypotheses. 2017 Jun;103:10–20.
-
22. Tian Z, Chang X-H, Zhao Y, Zhu H-L. Current biomarkers for the detection of endometriosis. Chin Med J (Engl). 2020 Aug 26;133(19):2346-52.
-
23. May KE, Conduit-Hulbert SA, Villar J, Kirtley S, Kennedy SH, Becker CM. Peripheral biomarkers of endometriosis: a systematic review. Hum Reprod Update. 2010 Dec;16(6):651–74.
-
24. Jee BC, Suh CS, Kim SH, Moon SY. Serum soluble CD163 and interleukin-6 levels in women with ovarian endometriomas. Gynecol Obstet Invest. 2008;66(1):47–52.
-
25. Hirsch M, Duffy J, Davis CJ, Nieves Plana M, Khan KS, International Collaboration to Harmonise Outcomes and Measures for Endometriosis. Diagnostic accuracy of cancer antigen 125 for endometriosis: a systematic review and meta-analysis. BJOG Int J Obstet Gynaecol. 2016 Oct;123(11):1761–8.
-
26. Mol BW, Bayram N, Lijmer JG, Wiegerinck MA, Bongers MY, van der Veen F, et al. The performance of CA-125 measurement in the detection of endometriosis: a meta-analysis. Fertil Steril. 1998 Dec;70(6):1101–8.
-
27. Maiorana A, Cicerone C, Niceta M, Alio L. Evaluation of serum CA 125 levels in patients with pelvic pain related to endometriosis. Int J Biol Markers. 2007 Jul-Sep;22(3):200-2.
-
28. Nisenblat V, Bossuyt PMM, Shaikh R, Farquhar C, Jordan V, Scheffers CS, et al. Blood biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016 May 1;(5):CD012179.
-
29. Chen Y, Zhu H-L, Tang Z-W, Neoh KH, Ouyang D-F, Cui H, et al. Evaluation of Circulating Endometrial Cells as a Biomarker for Endometriosis. Chin Med J (Engl). 2017 Oct 5;130(19):2339–45.
-
30. Ahn SH, Singh V, Tayade C. Biomarkers in endometriosis: challenges and opportunities. Fertil Steril. 2017;107(3):523–32.
-
31. Jia S-Z, Yang Y, Lang J, Sun P, Leng J. Plasma miR-17-5p, miR-20a and miR-22 are down-regulated in women with endometriosis. Hum Reprod Oxf Engl. 2013 Feb;28(2):322–30.
-
32. Burney RO, Hamilton AE, Aghajanova L, Vo KC, Nezhat CN, Lessey BA, et al. MicroRNA expression profiling of eutopic secretory endometrium in women with versus without endometriosis. Mol Hum Reprod. 2009 Oct;15(10):625–31.
-
33. Cho S, Mutlu L, Grechukhina O, Taylor HS. Circulating microRNAs as potential biomarkers for endometriosis. Fertil Steril. 2015 May;103(5):1252-1260.e1.
Articole din ediţiile anterioare
Impactul endometriozei asupra sarcinii
Endometrioza şi adenomioza sunt patologii care afectează frecvent femeile de vârstă reproductivă. Obţinerea şi menţinerea unei sarcini se fac de mu...
Rolul chirurgiei în cazul pacientelor infertile cu adenomioză
Adenomioza este o maladie benignă ce aparţine unui grup de afecţiuni estrogen-dependente, asociate cu unele consecinţe clinice semnificative: disme...