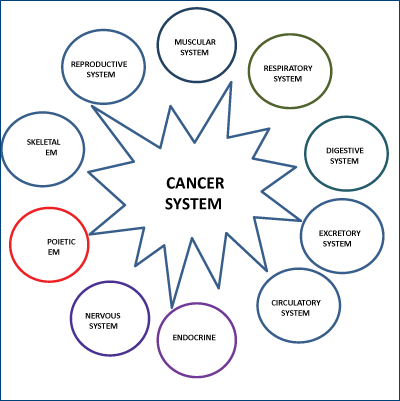
We introduce here for the first time the cancer system concept. This system consists of several geographically separated cancer tissues (the primary tumor, the local, and the distant metastasis), which form elaborate communicating networks between themselves. Although, the cancer system is initially derived from the normal organism, it gradually interferes with the normal functions, and ultimately it destroys the organism. According to the cancer type, stage, and location, the normal body systems become more or less involved and affected by the cancerous process. In order for the cancer cells to spread at distance, beyond the tissue of origin, they must do more than subvert the normal cells at the tissular level and induce them to serve “the neoplastic agenda”. These cancer cells also need to hack into other normal body systems (i.e. bone marrow stem cell reserve, vascular, lymphatic, immune, endocrine, nervous, etc) and modify these systems to promote the cancerous process. We strongly believe that in the near future a better understanding of the cancer process at the organismic level will lead to more efficient cancer treatment strategies.
Cancerul: imagine de ansamblu. Văzând pădurea dincolo de copaci
Cancer: the big picture. Seeing the forest beyond the trees
First published: 24 martie 2015
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/OnHe.30.1.2015.4305
Abstract
Rezumat
Vă prezentăm aici pentru prima dată conceptul de sistem al cancerului. Acest sistem este format din mai multe ţesuturi canceroase separate geografic (tumoare primară, locale, precum şi metastaze la distanţă), care formează reţele comunicatoare elaborate între ele. Deşi sistemul de cancer este derivat iniţial din organismul normal, treptat acesta interferează cu funcţiile normale şi în cele din urmă distruge organismul. În funcţie de tipul de cancer, stadiu şi localizare, sistemele normale a corpului devin mai mult sau puţin implicate şi afectate de procesul canceros. Pentru ca celulele canceroase să se răspândească la distanţă, departe de ţesutul de origine, ele trebuie să facă mai mult decât să submineze celulele normale la nivel tisular şi de a le determina să servească „pe ordinea de zi neoplazică”. Aceste celule canceroase de asemenea trebuie să penetreze alte sisteme normale ale organismului (de exemplu, rezerva de celule stem din măduva osoasă, vascular, limfatic, imunitar, endocrin, nervos etc.) şi să modifice aceste sistemele pentru a promova procesul canceros. Credem cu tărie că în viitorul apropiat o mai bună înţelegere a procesului canceros la nivel organismic va duce la strategii mai eficiente în tratamentul cancerului.
In the last few decades, an enormous body of literature has been published in an attempt to decipher the nature of cancer. Increasing evidence has been leading us to postulate that cancer is not just a simple lump of cells that divide, invade, and spread randomly, but rather a complex network of cells that communicate between themselves and create their own system, which ultimately take over the entire organism.
In a seminal article published in Cell in 2000(1), Hannahan and Weinberg stated that: “The field of cancer research has largely been guided by a reductionist focus on cancer cells and the genes within them (...). Looking forward in time, we believe that important new inroads will come from regarding tumors as complex tissues in which mutant cancer cells have conscripted and subverted normal cell types to serve as active collaborators in their neoplastic agenda. The interactions between the genetically altered malignant cells and these supporting conspirators will prove critical to understanding cancer pathogenesis and to the development of novel, effective therapies”. Basically, in a new approach to cancer treatment, the authors suggest the understanding of the malignant process from the tissular perspective. According to the above authors, cancer does not only consist of the genotypic and phenotypic transformation of normal cells into cancer cells, but it also consists of the appearance of new interactions between the cancer cells and the surrounding stromal cells, causing the formation of the cancer tissue. The cancer stroma consists of modified fibroblasts, immune cells, and endothelia cells, that are not passive bystanders but are active supporters of the cancer process. We propose a broader perspective and introduce the cancer system concept. This system consists of several geographically separated cancer tissues (the primary tumor, the local, and the distant metastasis), which form elaborate communicating networks between themselves. Although the cancer system is initially derived from the normal organism, it gradually interferes with the normal functions, and ultimately it destroys the organism. According to the cancer type, stage, and location, the normal systems become more or less involved and affected by the cancerous process. In order for the cancer cells to spread at distance, beyond the tissue of origin, they must do more than subvert the normal cells at the tissular level and induce them to serve “the neoplastic agenda”. These cancer cells also need to hack into other normal body systems (i.e. bone marrow stem cell reserve, vascular, lymphatic, immune, endocrine, nervous etc.) and modify these systems to promote the cancerous process (Figure 1).
The development of the cancer system is due to the appearance of a new blue print, at the cellular level, distinct from the blue print of the normal organism. Thus, in every patient with cancer there are two distinct cellular blue prints at work: the normal organism cellular blue print that regulates the development and functioning of the normal cells and the cancer organism cellular blue print that regulates the progression and the functioning of the cancer cells. These cellular blue prints are comprised of genetic and epigenetic codes, respectively(2). There is a fundamental difference between the nature of the two blue prints in the adult organism. Namely, the cancerous blue print is “open,” while the normal blue print is “closed”. The cancerous “open” blue print is able to mutate, change and adapt continuously in response to environmental challenges, in a sort of accelerated intra-organism microevolution(3), while the normal “closed” blue print does not change. The “openness” of the cancer blue print represents a yet unexplored hallmark of cancer. The idea of the cancer system and the existence of an interplay between the different cancerous tissues and the normal organism has been supported by several clinical and experimental observations. For example, clinically, it has been observed that there is a relationship between the primary tumor and the distal metastasis. In some cases of renal cell cancer, resecting the primary tumor induces a regression in the distal metastasis(4). On the contrary, in some experimental models of lung cancer, resecting the primary tumor may accelerate the development of the metastasis(5).
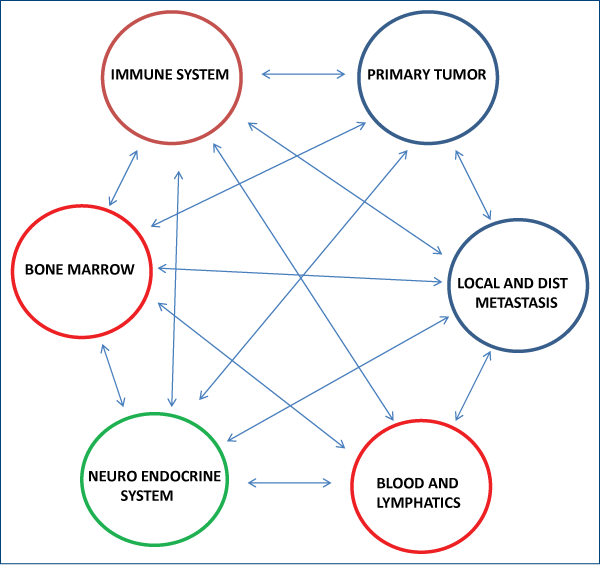
Charles Norton and Joan Massague have postulated since 2006 that circulating tumor cells (CTC) may re-infiltrate the tumor of origin enriching it with aggressive cells through the process of “tumor self-seeding”(6) and subsequent experimental data(7) supported their hypothesis. Further, recent evidence supports a link between tumors and the bone marrow, in which the bone marrow can function as a source of hematogenic progenitor cells that prepare the niche for metastasis(8) and, also possibly, as a source of malignant stem cells(9). Recent experiments suggest a direct relationship between the nervous system and certain tumors, as in stomach cancer, where denervation suppresses gastric tumorigenesis(10). Also, the role of different blood components(11,12) and the lymphatic system(13) in the metastatic process has been coming more and more into focus. Therapies targeted against blood and lymphatic factors involved in cancer are in development. A simplified version of the cancer system networks is shown below (Figure 2). The idea of a higher level of organization of cancer is not new. Recently, this paradigm has been pushed to its extreme implications by Mark Vincent from the University of Western Ontario. In a recent series of articles(14-16), the author describes cancer as a “protozoan-like organism” which appears due to the reactivation in the genome of an “ancient de-repressed survival program”. According to the author, cancer represents the return to a unicellular life form, a sort of regression to an “ancient and alternative organism, a living fossil, foreign to its host”. The core component of this “re-primitivization” process in which the cells return to an “ancient, de-repressed survival program” is, in Mark Vincent`s view, the metabolic switch to aerobic glycolysis (the Warburg phenomenon). Mark Vincent is not the first author to present the hypothesis of the “re-primitivization”. The same precise idea has been published in 1978 by Octavian Udrişte in his book “The ancestral gene and the origin of cancer”(17). Here is an excerpt: „6. The information about cancer is of endogeneous (endogenetic) origin, stored in a small fraction of the anaerobic DNA (ancestral), that is a natural component of the nuclear genome by all eucaryotes. 7. The cancer generating factors are antiinformational aggresors, originating in the external or internal medium (of the organism). They destroy information by entropy, derepressing (relieving) the oncogeneous genes from the anaerobic nucleic sequence and consiquently activates the whole ancestral cybernetic-data programme, while the specific cybernetic-data programme remains partially or completly inhibited.”
At present, most cancer treatments are directed towards the cancer cells (chemotherapy, tyrosine kinase inhibitors, genetic therapy using modified viral vectors, micro RNA-inhibitors, glycolytic pathway inhibitors, epigenetic agents etc.) or the cancer tissue (radiation, angiogenesis inhibitors immunotherapy, stromal cell inhibitors, stem cell inhibitors, etc). Most cellular and tissular therapies are prone to fail, because of the heterogeneous nature of cancer cells(18-22) and their “open” blue print.
There are very few treatments that specifically target cancer at the organismic level and disrupt the network of interactions between the primary tumor and respectively its metastasis, the bone marrow, the nervous system, the immune system etc. Cancer is a robust system that is able to maintain stable functioning despite various perturbations. The essential robustness of cancer is maintained through heterogeneous redundancy; i.e. the cancer tissue contains a heterogeneous distribution of genetically different cancer cells maintained by genetic instability(23). A holistic approach to cancer treatment may be less affected by cancer genetic instability and heterogeneity. We will provide one striking example that illustrates the power of the holistic approach. In 2011, a group of researchers from Switzerland, coordinated by Dr. Boris Pashe, treated a cohort of 41 patients diagnosed with advanced hepatocellular carcinoma using the intrabuccal administration of a device that was generating very low levels of an electromagnetic field which was amplitude modulated and adjusted at patient- specific frequencies. The results were very promising. 14 patients (34.1%) had stable disease for more than 6 months, the median progression-free survival was 4.4 months (95% CI 2.1-5.3) and the median overall survival was 6.7 months (95% CI 3.010.2). The treatment was very well tolerated and there were no toxicities associated with this treatment approach. The article was published in the prestigious “British Journal of Cancer”(24). The view of cancer as a multidimensional process with specific characteristics at the cellular, tissular and the organismic level, may represent a paradigm shift that may guide the way we understand and treat cancer in the immediate future.
In conclusion, there is a need for strategies that target specifically the cancer system and its networks. We strongly believe that in the near future a better understanding of the cancer process at the organismic level will lead to more efficient cancer treatment strategies.
Bibliografie
2. Paul, D. Taming Cancer Rom J Oncol Hematol. 2013; Vol. 1(1):8-10.
3. Kimmel, M. Evolution and cancer: a mathematical approach. Biology Direct 2010;5:29.
4. Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med 2001; 345 (23): 1655-9.
5. O’Reilly, M, Boehm T, Shing Y, et al. Endostatin: An Endogenous Inhibitor of Angiogenesis and Tumor GrowthCell, 1997; Vol. 88, 277–285.
6. Ki, M-Y, Oskarsson, T, Acharyya, S et al. ,Tumor self-seeding by circulating cancer cells. Cell. 2009 December 24; 139(7): 1315–1326.
7. Norton, L and Massague, J. Is cancer a disease of self-seeding? Nature medicine. 2006 12(8).875-878.
8. Mareel M, Oliveira MJ, Madani I.Cancer invasion and metastasis: interacting ecosystems. Virchows Arch. 2009; 454:599–622.
9. Kano, Y, Ishii, H, Konno M, et al. Cells of origin of squamous epithelium, dysplasia and cancer in the head and neck region after bone marrow transplantation. Int J Oncol. 2014 44(2):443-50.
10. Chun-Mei, Z, Hayakawa, Y, Kodama,Y, et al. Denervation suppresses gastric tumorigenesis. Sci Trans Medicine. 2014; Vol 6 (250): 1-12.
11. Beleva, E, Grudeva-Popova, J. From Virchow’s triad to metastasis: circulating hemostatic factors as predictors of risk formetastasis in solid tumors. J BUON. 2013; 18(1):25-33.
12. Krstic, J, Maslovaric, I, Santibanez, JF. Novel patents and cancer therapies for transforming growth factor-beta and urokinase type plasminogen activator: potential use of their interplay in tumorigenesis. Recent Pat Anticancer Drug Discov. 2014;9(3):354-71.
13. Karikoski, M, Marttila-Ichihara, F, Elima, K, et al. Clever -1/stabilin-1 controls cancer growth and metastasis. Clin Cancer Res. Volume 2014; 20(24):6452-64.
14. Vincent, M. The animal within: Carcinogenesis, and the clonal evolution of cancer
cells are speciation events sensu stricto. Evolution 2010; Vol. 64 (4): 1173–1183.
15. Vincent, M. Cancer: Beyond Speciation. Advances in Cancer Research 2011; 112: 283-350.
16. Vincent, M. Cancer: A de-repression of a default survival program common to all
cells? BioEssays 2012; 34(1):72-82.
17. Udrişte, O. Gena ancestrală şi originea cancerului, Editura ştiinţifică şi enciclopedică, Bucureşti, 1978.
18. Crockford, A, Jamal-Hanjani, M, Hicks, J and Swanton, C. Implications of intratumour heterogeneity for treatment stratification. J Pathol 2014; 232: 264–273.
19. Park, SY, Gönen, M, Kim, HJ, et al. Cellular and genetic diversity in the progression of in situ human breast carcinomas to an invasive phenotype. The Journal of Clinical Investigation 2010; Vol. 120 (2). 636-644.
20. Marusyk, A, Almendro, V and Polyak, K. Intra-tumour heterogeneity: a looking glass for cancer? Nature Reviews Cancer 2012; 12: 323-334.
21. Gerlinger,M, Rowan, AJ, Horswell, S et al. Intratumor Heterogeneity and Branched Evolution Revealed by Multiregion Sequencing. NEJM 2012; 366(10):883-891.
22. Wang, Y, Waters, J, Leung, M.L. et al. Clonal Evolution in Breast Cancer Revealed by Single Nucleus Genome Sequencing. Nature 2014; 512(7513): 155–160.
23. Kitano, H Nature Cancer as a robust system: implications for anticancer therapy. Nature Reviews Cancer 2004. 4 227-235.
24. Costa, FP, de Oliveira, Ac, Meirelles, R et al. Treatment of advanced hepatocellular carcinoma with very low levels of amplitude-modulated electromagnetic fields. British Journal of Cancer 2011; 105, 640-648.