The key element that has determined us to initiate a study concerning the benefits of intra-arterial chemotherapy is the fact that, despite progress regarding therapy, the ENT cancer prognosis is poor, especially in stages that have regional invasion, with a higher risk of therapeutic failure. ENT cancer treatment has raised many questions, the present therapeutic standard being simultaneous radio-chemotherapy with cisplatin, a quality regional control being obtained by hyper-fractionated radiotherapy, targeted on the tumour volume. The technical inconveniences that, in some cases, make impossible the accomplishment of a correct treatment, have determined us to make a retrospective analysis of the studies published in medical journals, with the aim of discerning a therapeutic method that has a role in increasing the loco-regional control of the disease. The studies speak about intra-arterial chemoinfusion with cisplatin, alongside radiotherapy, demonstrating with certainty the superiority of this method versus administrating the drug intravenously, in terms of local tumour control and tolerability. Because the benefits regarding the survival rate and the disease-free period have not reached the statistically significant level, intra-arterial chemoinfusion of cisplatin, alongside radiotherapy, remains a subject that is open to further studies. The efficacy of intra-arterial chemoinfusion of cisplatin hasn’t been studied in the case of a relapse, remaining still a subject that is worthy of being approached, seeing as this method was shown to be efficient regarding the safety profile, having a good tolerability and also having a good activity profile, leading to increase in the quality of life and an increase in survival through a local tumour control that is superior than in the case of intravenous administration of the drug.
Chimioterapia intraarterială a cancerelor sferei ORL
Intra-arterial chemotherapy of ENT cancers
First published: 17 mai 2017
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/OnHe.39.2.2017.728
Abstract
Rezumat
Elementul-cheie care ne-a determinat să inițiem un studiu privind evaluarea beneficiilor chimioterapiei intraarteriale este faptul că, în ciuda progreselor terapeutice, prognosticul cancerelor sferei ORL este rezervat, cu precădere în stadiile avansate locoregional cu risc crescut de a dezvolta eșecuri terapeutice. Tratamentul cancerelor sferei ORL a ridicat numeroase controverse, actual fiind stabilit ca standard terapeutic radio-chimioterapia concomitentă cu cisplatin, un control locoregional de calitate fiind obținut în cazul iradierii hiperfracționate, țintit pe volumul tumoral. Inconvenientele tehnice care, în anumite situații, fac imposibilă realizarea unui tratament corect ne-au determinat să realizăm o analiză retrospectivă a studiilor publicate în revistele de specialitate, cu scopul decelării unei metode terapeutice cu rol în creșterea controlului locoregional al bolii. Studiile fac referire la chimioinfuzia intraarterială cu cisplatin, concomitent cu radioterapia, demonstrându-se cu certitudine superioritatea acesteia comparativ cu administrarea intravenoasă în termenii controlului local tumoral și al tolerabilității. Întrucât beneficiile în termenii ratei de supraviețuire și ai intervalului liber de boală nu au atins pragul semnificației statistice, administrarea chimioinfuziei intraarteriale cu cisplatin concomitent cu radioterapia rămâne un subiect deschis evaluării studiilor viitoare. În boala recidivantă, eficacitatea chimioinfuziei intraarteriale cu cisplatin nu a fost studiată, însă rămâne un subiect demn de a fi abordat întrucât s-a demonstrat că metoda prezintă eficacitate din punct de vedere al profilului de siguranță, având o tolerabilitate bună, precum și al profilului de activitate, realizând o ameliorare a calității vieții și o prelungire a supraviețuirii prin controlul local tumoral superior administrării intravenoase.
Introduction
Cancers in the head and throat area are a heterogeneous group of tumours that include tumours which have an origin point in the oral cavity, the oropharynx, the rhino-pharynx, the hypo pharynx, the larynx, the salivary glands, the thyroid and the sinuses.
The essential evolutionary particularity is backed up by a concept that implies that prolonged exposure of the oral and pharynx mucosa to carcinogens is what favours the onset and development of multifocal mucosal anomalies. There is a 2-6% risk per year of a second tumour appearing in the head and neck area, synchronous or metachronous, which appears in approximately 20-40% of the long-term survivors, especially in weak patients, smokers and alcohol consumers(29).
Head and neck cancers are mostly found in male patients (male/female ratio: 2.5/1), older people, with an average age of 50 years old, the cancer incidence increasing with age(30).
Head and neck cancers represent 10-15% of all cancers and make up 4-5% of all cancer deaths. In the European Union, the incidence is 34.6 cases/100000 people per year, with a mortality of 13.7 cases/100000 people per year. Also, annually, worldwide, approximately 650000 patients are diagnosed with head and neck cancer and 350000 patients die because of this disease(31).
Because of this increasing incidence and mortality of malign tumours of the head and neck, it entails the necessity of knowing the cause of the growth of these parameters, focusing our attention on the ENT controversial cancer treatment. Despite therapeutic progress, even in the molecular and immunotherapy treatment era, ENT cancers have a poor prognosis, especially those that are in stages with loco-regional invasion, with a higher risk of therapeutic failure, the correct staging of the disease and a proper treatment for that stage being important.
The role of intra-arterial chemoinfusion
Choosing adequate therapy in the head and neck cancers, in loco-regional advanced stages, is a controversial subject, treatments having a success rate of 30%, leaving cosmetic and functional scaring which aren’t that well tolerated by patients with a poor performance status and other comorbidities, this patient category including alcohol consumers and chronic smokers.
MACH-NC meta-analysis (Meta-Analysis of Chemotherapy in Head and Neck Cancer), which was updated in 2004, comprising 93 trials which included 17346 patients, showed that only radio-chemotherapy done at the same time had a statistically significant benefit, regarding the 5-year survival rate of 6.5% (p<0.0001), this benefit being maintained in every potential clinical situation, including post-operatory radiotherapy (HR=0.80), definitive conventional radiation (HR=0.83), and modified fractionated radiation (HR=0.73). The maximum benefit was obtained in the case of radio-chemotherapy concomitant with cisplatin (0.75), versus another chemotherapy agent (HR=0.86)(11-13).
Neoadjuvant polychemotherapy has showed a minimal benefit regarding the 5-year survival rate of only 2.4% (HR: 0.96).
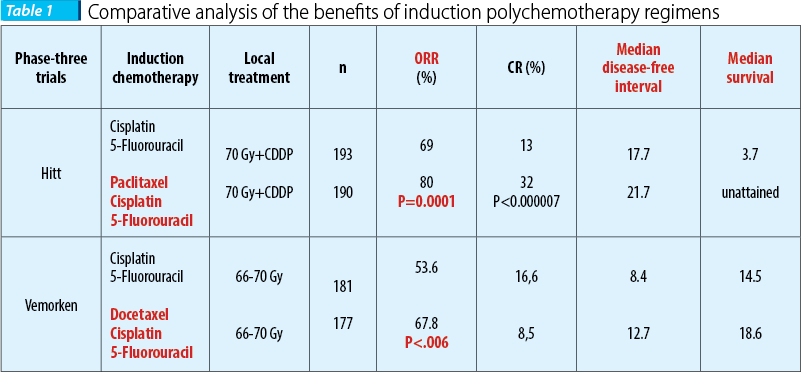
Still, the results of two phase-three randomized trials have shown that adding taxanes to cisplatin + 5-fluorouracil has a statistically significant benefit regarding the loco-regional control of the disease.
On a 384 patients lot with resectable disease vs. unresectable disease (66% of the patients), Hitt et al. compared the benefits of induction polychemotherapy based on taxanes regimen (paclitaxel 175 mg/sqm day 1 + cisplatin 100 mg/sqm day 2 + 5-fluorouracil 500 mg/sqm day 2-6) versus the standard regimen (5-fluorouracil + cisplatin), resulting in superior statistically significant results when adding paclitaxel, in terms of therapeutic response rate (69% vs. 80%, p=0.0001), with a cert improvement of general survival rate (26 months vs. 36 months) and of the disease-free interval (17.7 months vs. 21.7 months), preserving the organ being possible in a higher statistically significant percent (89% vs. 76%, p=0.046); the recorded toxicity was acceptable in both therapeutic regimens, with a more frequent mucosal toxicity grade III and IV for the standard regimen.
Similarly, in the EORTC 24971 phase-three randomized trial (European Organisation for Research and Treatment of Cancer), Vermoken et al. found that in patients with unresectable disease (stage III and IV) the polychemotherapy regimen based on taxans (docetaxel 75 mg/sqm day 1 + cisplatin 75 mg/sqm day 1 + 5-fluorouracil 750 mg/sqm day 1-5) versus the standard regimen (5-fluorouracil + cisplatin) was superior in terms of therapeutic response rate (53.6% vs. 67.8%, p=0.06), with an improvement in the survival rate (14.5 months vs. 18.6 months) and in the disease-free interval (8.4 months vs. 12.7 months); the toxicity was similar and acceptable in both therapeutic regimens, consisting of gastrointestinal toxicity, including nausea, emesis, stomatitis(14-19).
Controversies regarding neoadjuvant polychemotherapy are tied to the minimal benefits in terms of the survival rate and of the inherent toxicity, so that the natural question is if administering is justified or not, the answer being found in the results of the second phase-three randomized trial.
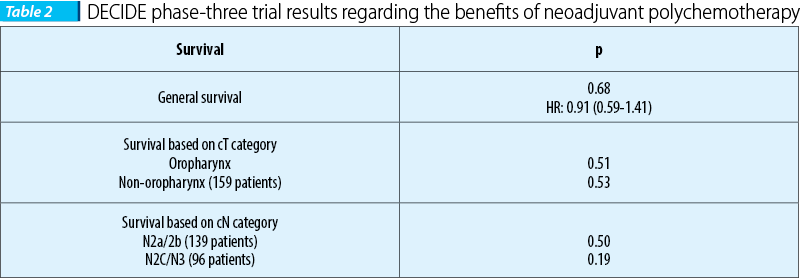
DECIDE, the first trial, included 280 patients with head and neck cancers and carcinoma of the oral cavity, hypopharinx and larynx, but especially oropharynx carcinomas (61.7%), loco-regional advanced stages and stages IVA and B (N2/3) respectively. The patients were homogenously randomized into two groups, the first one enlisting 142 patients which were administered neoadjuvant polychemotherapy, and two sequences of DPF regimen respectively, administered every 3 weeks (docetaxel 75 mg/sqm day 1, cisplatin 75 mg/sqm day 1, 5-fluorouracil 750 mg/sqm 120 hours) followed by concomitant radio-chemotherapy similar with the one used on the patients from the B group, that included 138 patients using the DFHX regimen (docetaxel 25 mg/sqm day 1, 5-fluorouracil 600 mg/sqm day 1-6, hydroxyurea 500 mg/12 hours, p.o. - day 1-6 concomitant with hyper-fractionated radiotherapy 1.5 Gy/fr, 2 fr/day, 5 days/week). Because there weren’t statistically significant differences in terms of the general survival rate, based on the clinical category T and N in favour of neoadjuvant polychemotherapy, administering it wasn’t justified(1-13).
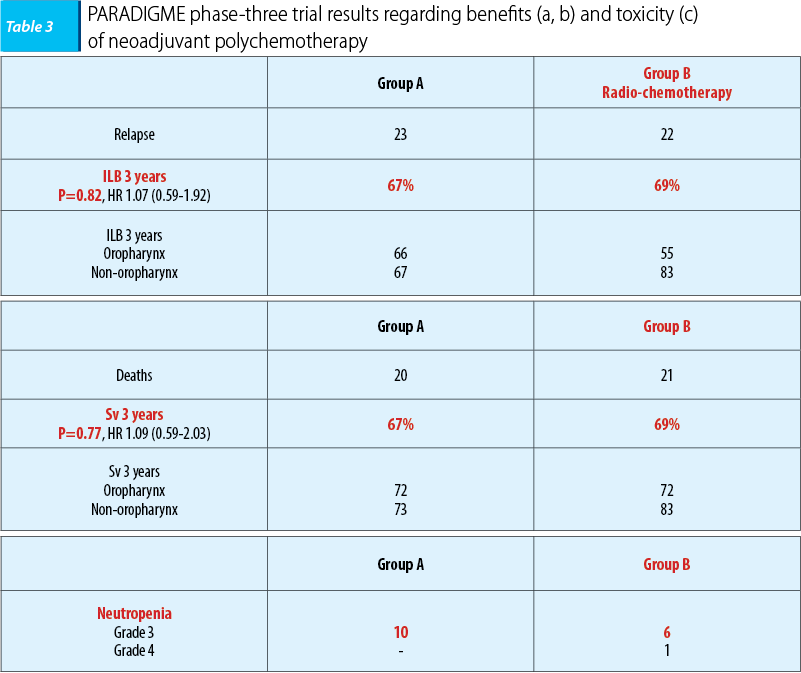
The second phase-three trial, PARADIGME, included 330 patients with ENT carcinomas and oral cavity, hypopharynx and larynx carcinomas respectively, the majority with oropharynx carcinomas (56%), the patients being randomized into two homogenous groups. Group A included 70 patients, which were administered neoadjuvant polychemotherapy, the same regimen (docetaxel 75 mg/sqm day 1, cisplatin 100 mg/sqm day, 5-fluorouracil 1000 mg/sqm, continuous perfusion days 1-4), followed by radio-chemotherapy concomitant with docetaxel on patients with stationary disease (docetaxel 20 mg/sqm/week 4 weeks, radiotherapy with the accelerated regimen - 72 Gy, 1.8 Gy/fr, 6 weeks), while those with full remission followed the first regimen with radio-chemotherapy concomitant with carboplatin (carboplatin AUC 1.5/week - 7 weeks, radiotherapy with the standard scheme - 70 Gy, 2 Gy/fr, 7 weeks). Group B included 75 patients who were administered radio-chemotherapy concomitant with cisplatin (cisplatin 100 mg/sqm/3 weeks, week 1 and 4, and radiotherapy the accelerated scheme - 6 weeks).
Statistically significant differences in terms of 3-year survival rate and 3-year disease-free interval between the two groups weren’t observed, the results being with 2 percent higher, in both situations, in the radio-chemotherapy concomitant with cisplatin group. Also, hematologic toxicity, and neutropenia respectively, were decreased in the radio-chemotherapy concomitant with cisplatin group. The lack of therapeutic benefits and the high toxicity are clear arguments that do not justify neoadjuvant poly-chemotherapy usage.
Controversies regarding concomitant chemotherapy and radiotherapy are tied to the benefits that erbitux brings relative to cisplatin.
The benefits of adding erbitux to cisplatin and radiotherapy (hyper-fractionated radiotherapy 72 Gy/40 fr/6 weeks, associated with cisplatin 100 mg/sqm/3 weeks and erbitux initially administered in doses of 400 mg/sqm/week and later in doses of 250 mg/sqm/week) were demonstrated in the Borner’s study. Comparing Bonner’s study results with those of MACH-NC meta-analysis (MetaAnalysis of Chemotherapy in Head and Neck Cancer), that investigated the absolute benefit in terms of survival regarding associating chemo-radiotherapy with cisplatin, the conclusion was that adding erbitux to radiotherapy leads to an absolute benefit for survival (10% at 5 years), similar with that obtained by adding cisplatin to radiotherapy (6.5% at 5 years). The results of this analysis recommend adding erbitux to radiotherapy, leading to an absolute benefit in terms of survival, representing an alternative to classic chemo-radiotherapy with a similar efficacy and an acceptable toxicity for head and neck cancers that are in a loco-regional advanced stage, with a high risk of local relapse and/or loco-regional (cT2-4 and cN2,3)(20-23).
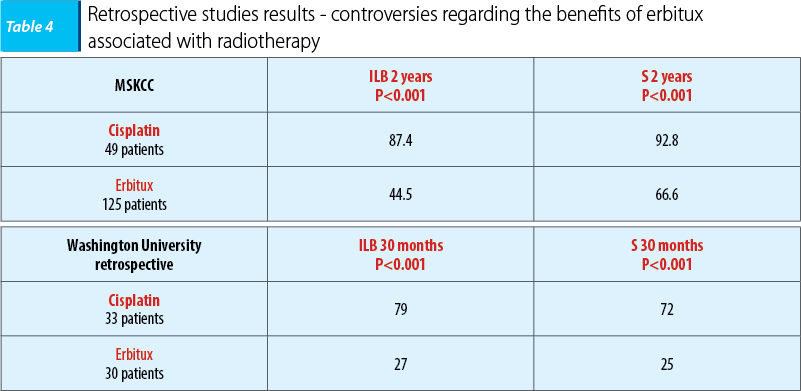
The benefits of erbitux concomitant with radiotherapy are controversial in the results of two retrospective studies, MSKCC (Koucher et al., Int J Radiat Oncol BioPhys 81: 915-922, 2011) and Washington University (Ley et al., Multidisciplinary Cancer Symposium Phoenix, AZ, January 26-28, 2012, Abst 163) respectively, a prospective study, TREMPLIN, being ongoing. The survival rate and disease-free interval benefits have reached the statistically significant level regarding concomitant radio-chemotherapy and cisplatin, results that clearly demonstrate the cisplatin superiority versus erbitux concomitant with radiotherapy.
But increasing loco-regional control of the disease remains the objective of hyper-fractionated radiotherapy, that demonstrated a benefit in terms of loco-regional control of the disease of 6% and of the survival rate of 3%, although with the risk of a severe toxicity that can be minimized when using targeted radiation, 3-D radiation or IMRT (intensity-modulated radiation therapy(24-27).
As such, the standard treatment of ENT carcinomas is concomitant radio-chemotherapy with cisplatin, a quality loco-regional control being obtained by using tumoral volume targeted hyper-fractionated radiation.
But radiotherapy ward overcrowding makes doing a hyper-fractionated radiation in all clinical cases impossible, and using standard fractionated radiation will lead to a decrease of the loco-regional control rate of the disease. Also, radiotherapy ward’s insufficient equipment makes impossible tumoral volume targeted radiation for all the patients that have been diagnosed with ENT carcinomas. But conventional radiation using wide radiation areas will certainly lead to a severe toxicity that imposes the ending of the treatment, and the prolonged display represents a loco-regional control rate decreasing factor.
These technical inconveniences have determined the authors of the study to make a retrospective analysis of the studies that were published in specialty journals with the aim of finding a therapeutic method with a role in increasing the disease loco-regional control. The published studies don’t mention other advanced radiation technics, but mention the role of chemotherapy with intra-arterial administration, in increasing loco-regional control, respective intra-arterial chemoinfusion, neoadjuvant administered, concomitant or in case of relapse.
Intra-arterial administration is made with the help of the angiograph and it presumes a femoral artery approach with carotid artery catheterization, the external carotid selectively and progressively the artery that vascularizes the tumour, where a catheter is fixed, that has been inserted with a guidewire through the femoral artery approach, catheter through which the cytostatic will be administered with the help of a chemotherapy injector that has been fixed in the arterial sheath.
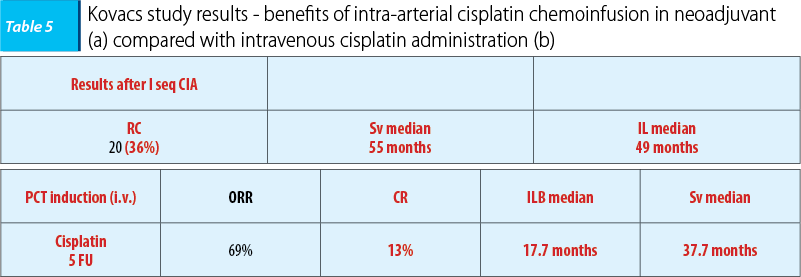
In 2004, Kovacs carried on a study that included 52 patients with carcinomas of the oral cavity and of the oropharynx, advanced loco-regional stages (stages III, IV A, B) and after a three year follow-up period, the study’s objective was finding the benefits of neo-adjuvant intra-arterial chemoinfusion, respective 1-2 cisplatin sequences in doses of 150 mg/sqm/3 weeks followed by i.v. sodium thiosulfate 9 g/sqm/30 minutes, followed by 12 g/sqm/12 hours, as a neutralizing agent of the renal toxicity of cisplatin. Compared with intravenous administration, the benefits were certain in terms of loco-regional disease control, complete remission rate being three times greater, with a positive impact regarding the patients’ prognostic with survival rate and disease-free interval improvement. Also, toxicity decreased, grade I/II, consisting especially of mucositis and gastrointestinal toxicity (nausea), remarkable being the fact that renal toxicity specific to intravenous administering of cisplatin wasn’t reported.
The conclusions of the study is that cisplatin intra-arterial neo-adjuvant chemoinfusion is superior to intravenous administration in terms of local tumoral control and of tolerability, but the results are limited to oral cavity and oropharynx carcinomas, and such as neo-adjuvant intravenous polychemotherapy, the method remains controversial, being recommended in advanced stages of the disease, when radiotherapy can’t be quickly initiated(28).
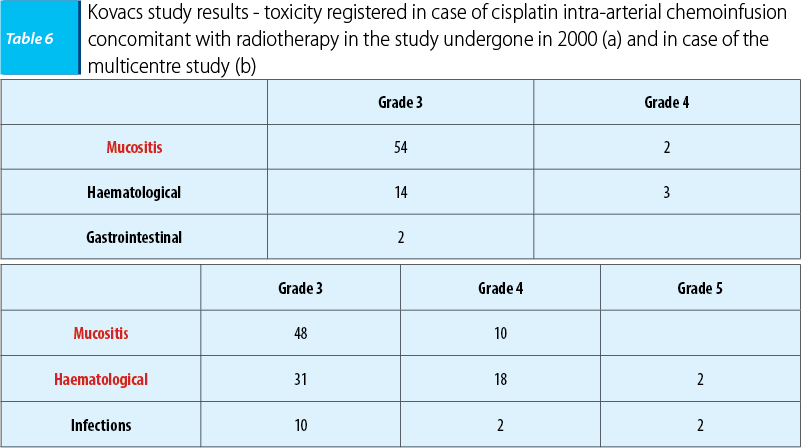
The first study that evaluated the benefits of cisplatin intra-arterial chemoinfusion concomitant with radiotherapy was that of Robbins, in 2000, that included 213 patients with ENT cancers in loco-regional advanced stages (stages III-IVA, IVB). Its promising results have determined the study’s extension in 2005 in the form of a prospective multicentre study - multiRADPLAT, that included 61 patients with oral cavity, oropharynx and hypopharynx carcinomas with a reserved prognosis, respectively stages IVA and IVB. The study’s objective was to reveal the benefits of cisplatin intra-arterial chemoinfusion concomitant with radiotherapy (1.8-2 Gy/fr DT 68-72 Gy), cisplatin being administered in doses of 150 mg/sqm/week, days 1, 6, 15, 22, being followed by intravenous sodium thiosulfate 9 g/sqm/30 minutes, followed by 12g/sqm/12 hours, as a cisplatin renal toxicity neutralizing agent. The results were similar in both studies with a loco-regional control rate of 80-90%, compared with 65-70% in case of cisplatin intravenous administration concomitant with radiotherapy, having an impact on patient prognosis by improving the disease free interval (74.3% compared with 39% in case of cisplatin intravenous administration), without finding benefits in terms of survival rate (53.6% compared with 54.6% in case of intravenous cisplatin administration). Because in the multicentre study the results regarding the survival rate and the disease-free interval were reported at 1-year and 2-year intervals, the results couldn’t be compared with those obtained in case of intravenous administration.
Also, toxicity was decreased in the 2000 initiated study (a), as well as in the multicentre study (b), consisting especially in mucositis, without reporting cisplatin-specific intravenous administration renal toxicity.
The conclusion of the studies was that cisplatin intra-arterial chemoinfusion concomitant with radiotherapy was superior to intravenous administration in terms of tumour local control and of tolerability(28).
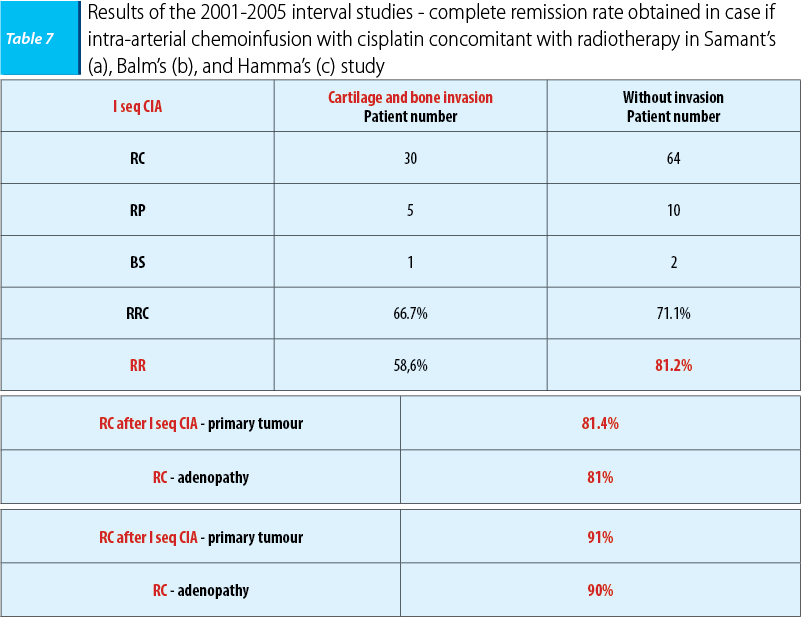
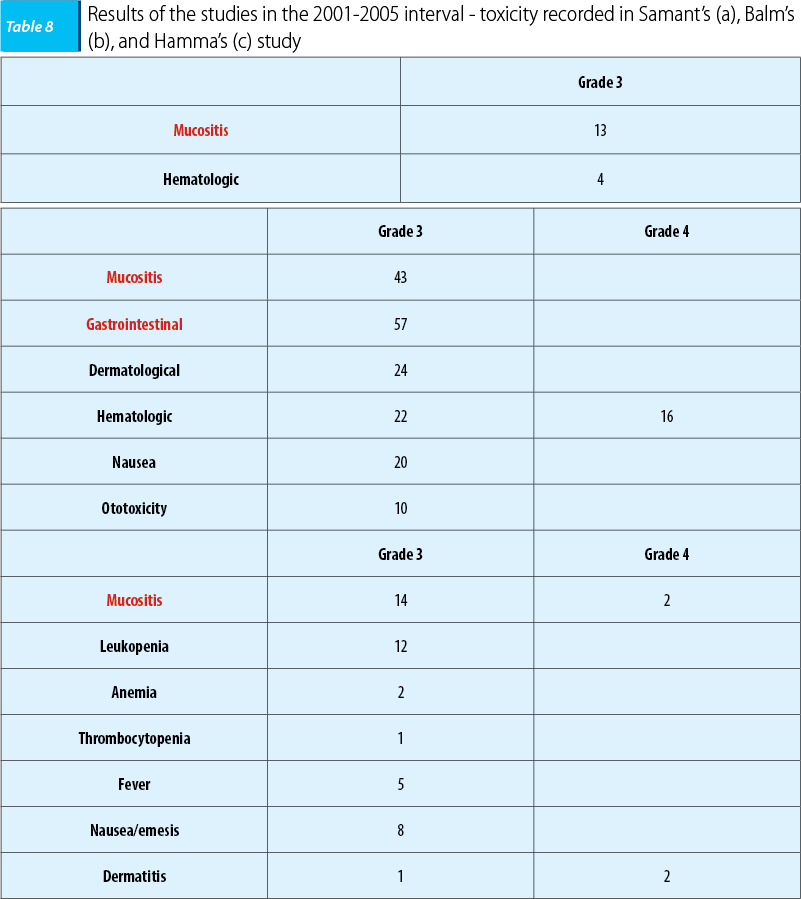
In the 2001-2005 interval, three studies were undergone that enrolled a large number of patients with ENT carcinomas with a poor prognosis in different clinic-imagistic states. In 2001, a study led by Samant enrolled 135 patients who presented or not imagistic cartilage and bone invasion, the patients being homogenously assigned. In 2004, the study led by Balm enrolled 79 patients with ENT carcinomas with a poor prognosis, in IVA and B stages respectively, and in 2005 the study led by Hamma enrolled 213 patients who featured imagistic invasion of the carotid artery, the prevertebral fascia, with/without metastasis presence at the time of the diagnosis. The objective of the study was revealing the benefits of cisplatin intra-arterial chemoinfusion concomitant with radiotherapy on patients with a poor prognosis and keeping the same therapeutic protocol, cisplatin administered in doses of 150 mg/sqm/weeks, in days 1, 6, 15, and 22. The benefits were certain in terms of disease loco-regional control with a complete remission rate of 80-90% in all situations.
None of the three studies reported data regarding benefits in terms of the survival rate and of the disease free interval, the only certain data being those tied to the loco-regional control of the disease.
Also, toxicity was decreased, consisting in every situation of mucositis and hematologic toxicity, cisplatin specific intravenous administration renal toxicity not being reported.
The conclusion of the studies was that intra-arterial chemoinfusion concomitant with radiotherapy was superior to intravenous administration in terms of tumoral local control and of tolerability(28).
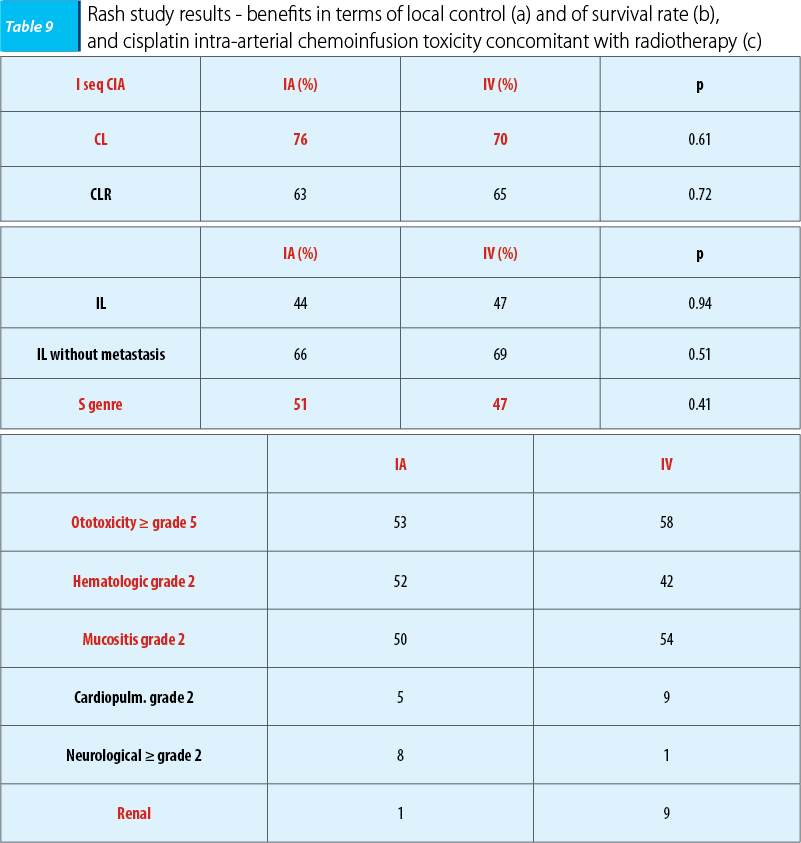
Similarly, in 2010, Rash initiated a phase-three trial which included 135 patients with ENT cancers in loco-regional advanced stages, respectively stages IVA and IVB. The objective was to evaluate the efficacy of cisplatin intra-arterial chemoinfusion compared with intravenous administration concomitant with radiotherapy, the patients being assigned homogenously into two lots. After a three-year follow-up, in the case of intra-arterial administration was found a minimal benefit in terms of tumoral local control and of the survival rate, but the treatment tolerability was good, the study’s conclusion being similar, namely that the patients with a poor prognosis intra-arterial chemoinfusion concomitant with radiotherapy was superior to intravenous administration in terms of tumoral local control and of tolerability(28).
Taking into account the renal toxicity of cisplatin when administered intravenously, a fact that imposes carboplatin administration, in 2007, Bertino made a study which enrolled 46 patients with ENT carcinomas, loco-regionally advanced cases, stages III-IVA and IVB, respectively. The results were mediocre, with a complete loco-regional remission rate of over 50%, so intra-arterial carboplatin chemoinfusion concomitant with radiotherapy was not justified because of the poor loco-regional control and because of the high mortality rate.
After the studies that evaluated benefits of intra-arterial chemoinfusion concomitant with radiotherapy, we can affirm that intra-arterial administered cisplatin is superior to intravenous administration in terms of the local control and of the tolerability.
Because the benefits in terms of survival rate and in terms of the disease free interval haven’t reached the statistically significant level, we can say that evaluating the efficacy of intra-arterial chemotherapy remains a subject open to analysis of future studies and would impose a comparative analysis of the benefits of intra-arterial administration compared with intravenous administration(28).
Still, there aren’t studies published in specialty literature that evaluate the benefits of intra-arterial chemoinfusion in case of relapse, a very important aspect taking into account that loco-regional advanced stages will develop in most cases, in the absence of a quality local control, relapses especially in the first two post-therapeutic years.
As such, initiating an individualized study regarding the therapeutic benefits of intra-arterial chemotherapy in loco-regional relapsed disease would be important, taking into account that it is the most crucial moment of local tumoral therapy, in which the therapeutic resources are exhausted, surgical intervention being mutilated and radiotherapy, even targeted, when the maximum admissible dose wasn’t administered, making possible a further radiation dose, has a limited benefit. Another justifying element is the results of the published studies that show with certainty the benefits of intra-arterial chemotherapy with regards to the tumoral local control and to tolerability. As a primordial element in case of relapse remains the local tumoral control taking into account that the presence of a tumour that is voluminous and compressive on vital structures can lead to complications with a vital risk. Also, in the relapsing disease, the tumour can bleed spontaneously leading to the possibility of recidivating epistaxis, of oral cavity bleeding, as well as of large vessel erosion of the neck region, leading to lethal haemorrhage through large vessel perforation (carotid artery, jugular vein). In these conditions, at the same time with the chemoinfusion embolization of the artery that vascularizes the tumour can be performed with the aim of interrupting the vascularization and stopping the haemorrhaging risk, as well as stopping the tumour evolution through hypoxia and necrosis secondary to vascularization interruption. We can say that in case of relapse it is important to apply intra-arterial chemoinfusion as it leads to far greater benefits compared to intravenous palliative chemotherapy administration. The obtained benefits concern quality of life improvement (severe and with a vital risk symptomatology improvement through epistaxis and haemorrhage stoppage and decrease of the complications secondary to the compression phenomenon due to the tumour) and prolonging survival because post embolization tumoral vascularization will be interrupted, leading to hypoxia and secondary necrosis with tumour evolution stoppage(28).
Conclusions
1. Standard treatment of ENT cancers in loco-regional advanced stages is represented by concomitant radio-chemotherapy which has proven a statistically significant benefit in terms of survival rate and of disease-free interval, the maximum benefit being attained in the case of cisplatin.
2. Neo-adjuvant poly-chemotherapy is recommended in case of advanced disease when radiotherapy can’t be quickly initiated.
3. Neo-adjuvant intra-arterial chemoinfusion is superior to intravenous administration in terms of local tumour control and of tolerability.
4. Neo-adjuvant intra-arterial chemoinfusion benefits are limited in case of oral cavity and oropharynx carcinomas and it remains a controversial therapeutic modality just like neo-adjuvant intravenous poly-chemotherapy, which is recommended in advanced disease when radiotherapy can’t be quickly initiated.
5. Intra-arterial chemoinfusion concomitant with radiotherapy is superior to intravenous administration in terms of local tumour control and of tolerability.
6. Intra-arterial chemoinfusion administered concomitant with radiotherapy hasn’t proved statistically significant benefits in terms of survival rate and of disease free interval, for which reason evaluating the benefits of this therapeutic method remains an subject open to future studies that will evaluate intra-arterial administration benefits compared with intravenous cisplatin administration.
7. Intra-arterial chemoinfusion is a safe method from a secure profile point of view, being simple, painless and easily tolerated.
8. Intra-arterial chemoinfusion is a safe method from an activity profile point of view, through quality of life improvement and prolonging survival through tumour evolution interruption.
9. Initiating an individualized study regarding therapeutic evaluation of intra-arterial chemotherapy benefits in case of loco-regional relapsed disease, in which therapeutic resources are exhausted, through mutilating surgery and limited radiotherapy, is important.
10. The major role of intra-arterial chemoinfusion in case of relapse is eliminating a vital risk (stopping epistaxis and haemorrhage), improving the symptomatology (reducing complications secondary to compression phenomenon) and prolonging survival (stopping the tumour evolution through hypoxia and necrosis, happening after the tumour vascularizing artery embolization).
Bibliografie
2. Wee J, Tan EH, Tai BC, Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union Against Cancer Stage III and IV nasopharyngeal cancer of the endemic variety, J Clin Oncol 2005; 23:6730-6738.
3. Mittal BB, Kepka A, Mahadevan A, Tissue/Dose Compensation to reduce toxicity from combined radiation and chemotherapy for advanced Head and Neck Cancers, Int J Cancer (Radiat Oncol Invest) 2001; 96:61-70.
4. Jin-Ching Lin, Jian-Sheng Jan, Chen-Yi Hsu, Phase III Study of Concurrent Chemoradiotherapy versus Radiotherapy alone for advanced nasopharyngeal carcinoma: Positive effect on overall and progression-free survival, J Clin Oncol 2003; 21:631-7.
5. Chan ATC, Teo PML, Ngan RK. Concurrent chemotherapyradiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: progression-free survival analysis of a phase III randomized trial, J Clin Oncol 2002; 20:2038–44. Pignon JP,
6. Bourhis J, Domenge C, Designe L. Chemotherapy added to locoregional treatment for head and neck squamous cell carcinoma: three meta-analyses of updated individual data. MACH-NC collaborative group. Metaanalysis of chemotherapy on head and neck cancer, Lancet 2000; 355:949–55.
7. Bourhis J, Armand JP, Pignon JP. Update of MACH-NC (Meta-Analysis of Chemotherapy in Head and Neck Cancer) database focused on concomitant chemoradiotherapy, Proc Am Soc Clin Oncol 2004; 22 [abstract5505].
8. Forastičre AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer, N Engl J Med 2003; 349:2091–8.
9. Denis F, Garaud P, Bardet E. Final results of the 94-01 French Head and Neck Oncology and Radiotherapy Group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced-stage oropharynx carcinoma, J Clin Oncol 2004; 22:69-76. Epub 2003 Dec 02.
10. Bernier J, Domenge C, Eschwege F, et al. Chemoradiotherapy, as compared to radiotherapy alone, significantly increases disease-free and overall survival in head and neck patients after surgery: results of EORTC phase III trial 22931 [abstract]. Int J Radiat Oncol Biol Phys 2001; 51:1.
11. Jeremic B, Shibamoto Y, Milicic N. Hyperfractionated radiation therapy with or without concurrent low-dose daily cisplatin in locally advanced squamous cell carcinoma of the head and neck: A prospective randomized trial, J Clin Oncol 2000; 18:1458-1464.
12. Pignon JP, Bourhis J, Domenge C, Designe L. Chemotherapy added to locoregional treatment for head and neck squamous cell carcinoma: three meta-analysis of updated individual data, Lancet 2000; 355:949–55
13. Overgaard J, Hansen HS, Specht L. The Danish Head and Neck Cancer Study group DAHANCA 6&7 randomized trial of 5 versus 6 fractions per week of conventional radiotherapy of squamous cell carcinoma of the head and neck, Proceedings 39th Annual Meeting of the American Society of Clinical Oncology (ASCO), Chicago, IL, May 31 - June 3, 2003.
14. Posner M, Colevas A, Tischler RB. The role of inction chemotherapy in the curative treatment of squamous cell cancer of the head and neck, Semin Oncol 2000; 27:13–24.
15. Ghi MG, Paccagnella A, D’Amanzo P, Mione CA, S, Paro S, Mastromauro C, Carnuccio R, Turcato G, Gatti C, Pallini A, Nascimben O, Biason R, Oniga F, Medici M, Rossi F, Fila G. Neoadjuvant docetaxel, cisplatin, 5-fluorouracil before concurrent chemoradiotherapy in locally advanced squamous cell carcinoma of the head and neck versus concomitant chemoradiotherapy: a phase II feasibility study, Int J RadiatOncolBiol Phys., 2004 Jun 1; 59(2): 481.
16. Gibson MK, Li Y, Murphy B. Randomized phase III evaluation of cisplatin plus fluorouracil versus cisplatin plus paclitaxel in advanced head and neck cancer (E1395): an intergroup trial of the Eastern Cooperative Oncology Group, J ClinOncol 2005; 23:3562-3567.
17. G, Kritselis G, Liossi P, Giannakouras G, Douzinas E, Katsilieris I. Induction chemotherapy followed by concurrent chemoradiation in advanced squamous cell carcinoma of the head and neck: final results from a phase II study with docetaxel, cisplatin and 5-fluorouracil with a four-year follow-up, Oral Oncol. 2006 Aug; 42(7):675-84. Epub 2006 May 30.
18. JB Vermorken, E Remenar, C van Herpen, J Germalluch, S Stewart, T Gorlia, M Degardin, K Schollen, J Bernier. Standard cisplatin/infusional 5-fluorouracil (PF) vs docetaxel (T) plus PF (TPF) as neoadjuvant chemotherapy for nonresectable locally advanced squamous cell carcinoma of the head and neck (LASCCHN):a phase III trial of the EORTC Head Neck Cancer Group (EORTC #24971) (abstract), ASCO Annual Meeting Proceedings (post-meeting edition), J ClinOncol 2004; 22:5508.
19. Posner M, Colevas A, Tischler RB., The role of induction chemotherapy in the curative treatment of squamous cell cancer of the head and neck, Semin Oncol 2000; 27:13–24.
20. Bonner JA, Giralt J, Harari PM. Cetuximab prolongs survival in patients with locoregionally advanced squamous cell carcinoma of head andneck: A phase III study of high dose radiation therapy with or without cetuximab [abstract], J ClinOncol, 2004; 22(14S): 5507.
21. Bonner JA, Harari PM, Giralt J. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck, N Engl J Med. 2006; 354 (6): 567-578.
22. Chan AT, Hsu MM, Goh BC, Multicenter, phase II study of cetuximab in combination with carboplatin in patients with recurrent or metastatic nasopharyngeal carcinoma, J Clin Oncol 2005; 23:3568-3576.
23. Trigo J, Hitt R, Koralewski P, Cetuximab monotherapy is active in patients (pts) with platinum-refractory recurrent/metastatic squamous cell carcinoma of the head and neck (SCCHN): Results of a phase II study (abstract). ASCO Annual Meeting Proceedings (post-meeting edition), J Clin Oncol 2004; 22:5502.
24. Etessami A, Lapeyre M, Tortochaux J, Geo ffrois L, Verrelle P, Domenge C, Wibault P, Auperin A, Bourhis J. Very accelerated RT versus accelerated RT + concomitant CDDP-5 FU in patients with palpable N2 and N3HNSCC: preliminary results of the GORTEC 96-01 randomize trial, Lung Cancer 2001;34[suppl 1], S68.
25. Abitbol A, Abdel-Wahab M, Lewin A, Troner M, Rodrigues MA, Hamilton-Nelson KL, Markoe A, Phase II study of tolerance and efficacy of hyperfractionated radiotherapy and 5-fluorouracil, cisplatin, and paclitaxel (Taxol) in stage III and IV inoperable and/or unresectable head-and-neck squamous cell carcinoma: A-2 protocol, Int J RadiatOncolBiol Phys. 2002 Jul 15; 53(4):942-7.
26. Sakata K, Oouchi A, Nagakura H, Akiba H, Tamakawa M, Koito K, Hareyama M, Asakura K, Satoh M, Ohtani S. Accelerated radiotherapy for T1,2 glottic carcinoma: analysis of results with KI-67 index, Int J Radiat Oncol Biol Phys 2000; 47:81–8.
27. Hliniak A, Gwiazdowska B, Szutkowski Z, Kraszewska E, Kukolowicz P, Jarzabski A, A multicentre randomized/controlled trial of a conventional versus modestly accelerated radiotherapy in the laryngeal cancer: influence of a 1 week shortening overall time, Radiother Oncol 2002; 62:1–10.
28. Locoregional Tumor Therapy – Editors: Eric Van Cutsem, Thomas J. Vogl, Franco Orsi, Alberto Sobrero.
29. Califano J, van der Riet P, Westra W. Genetic progression model for head and neck cancer: implications for field cancerizatio, Cancer Res 1996, 56:2488–2492.
30. Smith E.M., Ritchie J.M., Summersgill K.F., Age, sexual behavior and human papillomavirus infection in oral cavity and oropharyngeal cancers, Int J Cancer, 108, 766+772, 2004.
31. Ferlay J, Bray F, Pisani P. Cancer incidence, mortality and prevalence wordwide, version 1.0 (IARC Cancer Base No 5), Lyon: IARC Press, 2001.
Articole din ediţiile anterioare
Pacienţii cu tumori maligne - intervenţie psihologică
Data from the literature reveals that a person with cancer may be exposed to a wide range of physical, emotional, psychological, social and practic...
Cancerul de canal anal - aspecte legate de diagnostic și tratament
Cancerul anal reprezintă o neoplazie rară, constituind aproximativ 1,5% din totalitatea cancerelor digestive, dar rămâne o preocupare deosebită din...
Studiul erorilor diagnostice și terapeutice corelate cu studiul markerilor tumorali cu impact în prognosticul pacienților cu cancer localizat la cap și gât
Introducere. Alegerea protocolului de tratament pentru pacienții cu carcinoame ale sferei ORL stadii avansate locoregional, rămâne o discuție desc...