Background. During the novel coronavirus pandemic, the median overall survival of lung cancer patients has decreased from 7.9 months to 6.7 months. In the TERAVOLT analysis of patients with COVID-19 and thoracic cancers gathered from eight countries, 33% died and 76% needed hospital admission, with an age above 65 years old and presence of any comorbidities being negative prognostic factors. Lung cancer remains a leading cause of cancer-related death worldwide, despite the progress made with targeted therapies and immunotherapy. We present the case of an elderly patient diagnosed with metastatic pulmonary adenocarcinoma who, despite cardiovascular comorbidities and a negative expression of PD-L1, showed response to nivolumab (anti-PD-1) therapy and had a good clinical outcome after SARS-CoV-2 infection. Methodology. In January 2019, an 80-year-old female was diagnosed with acinar-predominant adenocarcinoma of the lung, stage IVA, with lung metastasis. She underwent surgical treatment, consisting of atypical resection of the left inferior lobe. The tumor tested negative for ALK/EGFR mutations and PD-L1 expression. The patient had a history of treated thyroid neoplasm and several cardiovascular disorders. Due to her good performance status, she received first-line chemotherapy with a platinum-based regimen and bevacizumab for one year, until progression was noted following the imagistic evaluation by computed tomography (CT). Immunotherapy with nivolumab was started in February 2020. Results. Under nivolumab therapy, the tumor showed an initial pseudoprogression, followed by partial response, according to iRECIST criteria. The patient tolerated well the treatment. In December 2020, she tested positive for SARS-CoV-2 infection, with severe symptoms and CT findings of bilateral ground-glass opacities. After specific treatment, with favourable outcome, she was able to continue the nivolumab therapy. Discussion. Anti-PD-L1/PD-1 antibodies (e.g., nivolumab) act by binding on the programmed death-1 (PD-1) receptor found on T cells and blocking its interaction with its ligands, programmed death ligand 1 and 2 (PD-L1 and PD-L2). Those ligands mediate an inhibition of active T-cell proliferation and surveillance of tumors. Tumors can efficiently evade immune responses by expressing PD-L and activating this negative regulatory pathway. Although the expression of programmed death ligand 1 (PD-L1) on tumor cells is used in clinical practice as a predictive biomarker for the response to the therapy with anti-PD-L1/PD-1 antibodies, histopathological examinations revealed several examples of how PD-L1 expression can be a misleading marker. It is possible to have a good response to the aforementioned therapy in a PD-L1 negative patient or a lack of response in a PD-L1 positive one. Conclusions. This case illustrates the management of an elderly cancer patient with lung cancer and SARS-CoV-2 infection. The favourable response to nivolumab therapy reinforces the need for new biomarkers and more accurate testing assays in the clinical practice, in order to accurately predict the tumor response to anti-PD-1 antibodies.
Fighting disease at 83 years old: SARS-CoV-2 survival and response to anti-PD-1 therapy in PD-L1 negative adenocarcinoma of the lung – case report
Luptând cu boala la 83 de ani: supravieţuire în cazul infecţiei cu SARS-CoV-2 la o pacientă cu adenocarcinom pulmonar PD-L1 negativ, care a răspuns la tratament anti-PD-1 – prezentare de caz
First published: 25 martie 2022
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/OnHe.58.1.2022.6229
Abstract
Rezumat
Context. În timpul pandemiei de COVID-19, supravieţuirea totală medie a pacienţilor cu cancer pulmonar a scăzut de la 7,9 luni la 6,7 luni. În analiza TERAVOLT a pacienţilor cu COVID-19 şi cancer toracic din opt ţări, 33% au murit şi 76% au avut nevoie de internare în spital, iar vârsta mai mare de 65 de ani şi prezenţa oricăror comorbidităţi au fost factori de prognostic negativ. Cancerul pulmonar rămâne, la nivel mondial, una dintre principalele cauze de deces cauzat de o neoplazie, în ciuda progreselor înregistrate cu terapiile ţintite şi de imunoterapie. Prezentăm cazul unei paciente de 83 de ani, diagnosticată cu adenocarcinom pulmonar metastatic, care, în ciuda comorbidităţilor cardiovasculare şi a unei expresii negative a PD-L1, a răspuns la terapia cu nivolumab (anti-PD-1) şi s-a recuperat bine după infecţia cu SARS-CoV-2. Metodologie. În ianuarie 2019, o femeie în vârstă de 80 de ani a fost diagnosticată cu adenocarcinom pulmonar cu predominanţă acinară, stadiul IVA, cu metastaze pulmonare. S-a decis rezecţia atipică a lobului inferior stâng. Testele efectuate pe ţesutul tumoral au ieşit negative pentru mutaţiile ALK/EGFR şi expresia PD-L1. Pacienta avea antecedente de neoplasm tiroidian tratat şi mai multe afecţiuni cardiovasculare. Datorită statusului bun de performanţă, a primit chimioterapie de primă linie cu un regim pe bază de platină şi bevacizumab timp de un an, până când a fost observată progresia în urma evaluării imagistice prin tomografie computerizată (CT). În februarie 2020 a fost iniţiată imunoterapia cu nivolumab. Rezultate. Sub terapia cu nivolumab, tumora a prezentat o pseudoprogresie iniţială, care a fost însă urmată de un răspuns parţial, conform criteriilor iRECIST. Pacienta a tolerat bine tratamentul. În decembrie 2020, în urma unor simptome sugestive severe, testul SARS-CoV-2 al pacientei a ieşit pozitiv, iar examinarea CT a evidenţiat opacităţi bilaterale în geam mat. După tratamentul specific, pacienta a evoluat favorabil, putând continua terapia cu nivolumab. Discuţie. Anticorpii anti-PD-L1/PD-1 (ex.: nivolumab) acţionează prin legarea de receptorul programmed death-1 (PD-1) aflat pe celulele T şi blochează interacţiunea acestuia cu liganzii săi, programmed death ligand 1 şi 2 (PD-L1 şi PD-L2). Aceşti liganzi mediază o inhibare a proliferării celulelor T şi a capacităţii lor de a ţine sub control procesele neoplazice. Tumorile pot evita răspunsurile imune prin exprimarea PD-L la suprafaţa celulelor şi activarea acestei căi inhibitoare. Deşi expresia programmed death ligand 1 (PD-L1) pe celulele tumorale este utilizată în practica clinică ca biomarker predictiv pentru răspunsul la terapia cu anticorpi anti-PD-L1/PD-1, examinările histopatologice, cât şi experienţa clinică au dezvăluit câteva exemple în care expresia PD-L1 poate fi un marker înşelător. Astfel, este posibil să existe un răspuns bun la terapia menţionată anterior la un pacient cu un neoplasm care apare negativ pentru expresia PD-L1 (chiar şi excluzând cazurile în care testul iese fals negativ). De asemenea, poate exista lipsa unui răspuns la tratament în cazul unui pacient PD-L1 pozitiv. Concluzii. Acest caz ilustrează evoluţia sub tratament a unei paciente vârstnice, cu cancer pulmonar PD-L1 negativ şi infecţie cu SARS-CoV-2. Răspunsul favorabil la terapia cu nivolumab este o nouă dovadă că există nevoia de noi biomarkeri şi de teste mai precise în practica clinică şi care să poată prezice cu exactitate răspunsul tumorii la anticorpii anti-PD-1.
Background
During the novel coronavirus pandemic, the median overall survival of lung cancer patients has decreased from 7.9 months to 6.7 months(1).
Old age, cardiovascular disease and cancer are among the most important negative prognosis factors for mortality or severe disease in patients with COVID-19 infectious disease(2,3).
In the TERAVOLT analysis of patients with COVID-19 and thoracic cancers gathered from eight countries, 33% died and 76% needed hospital admission, with an age above 65 years old and the presence of any comorbidities being negative prognostic factors(4). In a review by Aran et al., the case fatality rate of COVID-19 in the overall population was 2.3%, whereas, when considering patients with malignancies alone, the rate doubled (5.6%)(5).
Following those observations, the mechanisms underlying the negative influence of the association of neoplasm and SARS-CoV-2 infection have been discussed – on the one hand, cancer patients could be associated with decreased immune surveillance and, therefore, with an altered ability to fight infection and, on the other hand, the inflammation associated with the novel coronavirus may even stimulate tumor growth, a process called pro-tumour inflammation(6).
Regarding the impact of age on the prognosis of the disease, the overall fatality rate of coronavirus disease in Romania was 2.6%, according to the official data available on the 23rd of April 2021(7). Compared to that, in an important meta-analysis performed by Verity et al., the fatality of the SARS-CoV-2 infection in persons older than 80 with more than one comorbidity was 13.4%(8).
Lung cancer remains a leading cause of cancer-related death worldwide(9), despite the progress made with targeted therapies and immunotherapy. Efforts are made to improve those treatments even further, and the selection criteria used for patients that could benefit from immune checkpoint inhibitors (ICI), such as nivolumab, are still to be improved. One of those criteria is the surface expression of programmed death ligand 1 (PD-L1) on the tumor cells(10). Adenocarcinoma is the most common lung neoplasm subtype to be diagnosed in never-smoker individuals and the most prevalent non-small cell cancer. The mean age of diagnosis is 71 years old, but there is an increased need to study the appropriate therapy recommendations in patients over 80(11,12).
We present the case of an elderly patient diagnosed with metastatic pulmonary adenocarcinoma who, despite cardiovascular comorbidities and a negative expression of PD-L1, showed response to nivolumab (anti-PD-1) therapy and had a good clinical outcome after SARS-CoV-2 infection.
Methodology
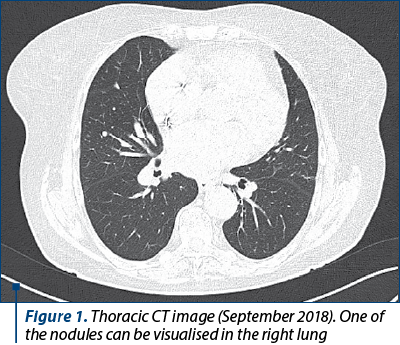
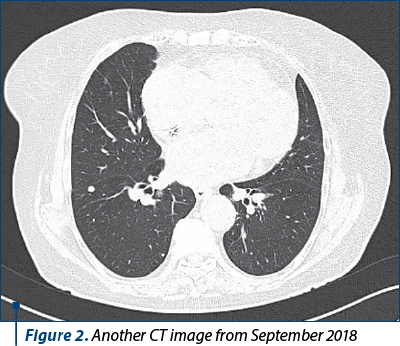
In May 2018, a 79-year-old female patient with multiple cardiovascular comorbiditiesa and a history of thyroid neoplasmb presented to the hospital with dyspnea, productive cough with blood in sputum and fever, being admitted for a pneumonia suspicion. A thoracic computed tomography (CT) was performed that revealed multiple nodular and micronodular opacities in all lobes of both lungs, which were, at that moment, interpreted as a pulmonary recidivation of the previous cancer (Figures 1 and 2).
In December 2018, an atypical resection of the left inferior pulmonary lobe was performed and the histopathological examination revealed an acinar-predominant adenocarcinoma of the lung, TxNxM1a (stage IV A). The tumor tested negative for ALK/EGFR mutations and PD-L1 expression.
The patient was normoponderal, had never smoked and had worked as a Persian rug weaver.
The ECOG performance status was 1 and the laboratory analyses were within the limits of normal values. Due to her good performance status, she received first-line chemotherapy with a platinum-based regimen and bevacizumab for one year (February 2019 – February 2020): 14 series with 250 mg of paclitaxel, 450 mg of carboplatin and 480 mg of Avastin® (bevacizumab), combined with pegfilgrastin.
Despite the treatment, progression was noted on CT, according to iRECIST criteria, and in February 2020 the patient started the treatment with nivolumab, 240 mg once every two weeks.
Results
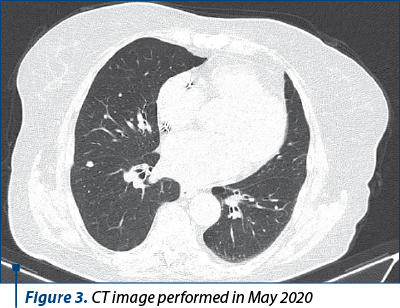
The treatment was tolerated well. Following the initiation of immunotherapy, an abdominal, pelvic and thoracic CT performed in May 2020 showed a pseudoprogression(13) of the pulmonary nodules (Figure 3).
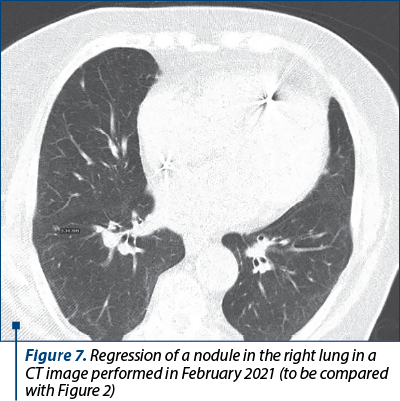
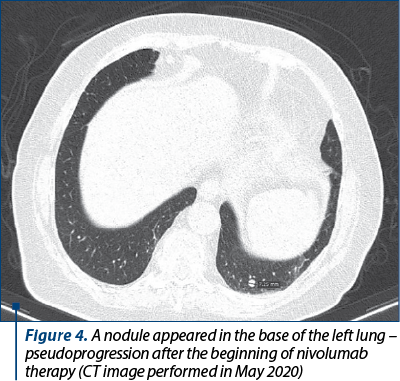
In September 2020, a new abdominal, pelvic and thoracic CT showed partial response to treatment – regression of some of the nodules in both lungs, which was successfully maintained since then under ongoing nivolumab therapy. Currently, she has continued the treatment with nivolumab for 26 seriesc (Figure 4).
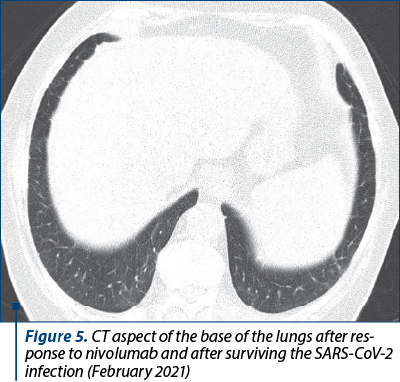
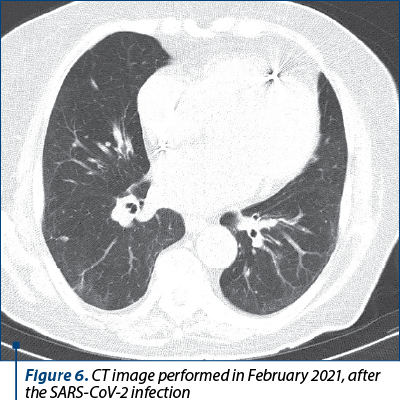
In December 2020, the patient was admitted for fever, worsening of the basal dyspnea to dyspnea in rest and thoracic pain. She tested positive for SARS-CoV-2 infection. The thoracic CT demonstrated bilateral ground-glass opacities and the complete blood count showed hypochromic microcytic anemia. Following admission, the patient was given antiviral treatment (favipiravir), corticotherapy and novel anticoagulants and she was stabilized with oxygenotherapy and erythrocyte mass transfusion.
After two weeks, her symptoms had significantly improved and she was discharged from the hospital. The ECOG performance status changed from 1 to 3 following the coronavirus infection and the NYHA dyspnea score changed from 2 to 3. However, she was able to continue the nivolumab therapy.
We present a series of CT images of the patient’s lungs that show the evolution of the pulmonary nodules under treatment and after the coronavirus infection.
Discussion
This case illustrates the management of an elderly cancer patient with lung cancer and SARS-CoV-2 infection. The tumor partially responded to immunotherapy with PD-1 antibody, even though it was negative for surface expression of PD-L1.
A major success of immunotherapy was finding evidence that the use of ICI resulted in a more prolonged long-term survival than the conventional anticancer drugs(14). The first ICI approved for treating lung cancer was nivolumab, in 2014. This drug acts by binding on the programmed death-1 receptor found on T cells and blocking its interaction with its ligands, programmed death ligand 1 and 2 (PD-L1 and PD-L2). Those ligands mediate an inhibition of active T-cell proliferation and surveillance of tumors(15). Tumors can efficiently evade immune responses by expressing PD-L and activating this negative regulatory pathway(16). A tumor can be positive or negative for PD-L1 expression through a number of biological processes which have different consequences regarding the response to ICI therapy. These processes are:
A. Constitutive PD-L1 expression, independent of T cell presence.
B. Induced PD-L1 expression, determined by interferon-g production by T cells.
C. Absence of PD-L1 expression, an areactive consequence of the absence of T cells.
D. Constitutive inability to express PD-L1, even upon T cell infiltration.
The histopathological examination of the tumor can report all the four combinatory variants of PD-L1 and T cell presence. If a PD-L1 negative tumor not surrounded by T cells is eventually infiltrated with T cellsd and stimulated by interferon g, it may express PD-L1, thus becoming sensitive to ICI therapy. This is an example of a situation that could lead to ICI response in a patient that tested negative for PD-L1 expression. An interesting aspect is that, in the absence of T cells, there should not be expected a response to ICI, even in PD-L1 positive patients.
However, the expression of PD-L1 on tumor cells is still often used in clinical practice as the only predictive biomarker for response to nivolumab. Several studies have attempted to develop new biomarkers in order to improve the accuracy of the prediction – e.g., tumor mutation burder and tumor microenvironment (TME)-based biomarkers(17,18). On the other hand, efforts are made to find testing assays that are more accurate for determining the PD-L1 expression than the traditional immunohistochemistry, such as exosomal PD-L1 testing(19).
Conclusions
This case illustrates the management of an elderly cancer patient with lung cancer and SARS-CoV-2 infection who, despite having various negative prognosis factors, had a good clinical outcome. At the same time, this case is an example of a favourable response to nivolumab therapy in a PD-L1 negative patient. Such cases reinforce the need for implementing new biomarkers and more accurate testing assays in the clinical practice, in order to accurately predict tumor response to anti-PD-1 antibodies.
Conflict of interests: The authors declare no conflict of interests.
d For example, under treatment with Ipilimumab – an anti-CTLA-4 antibody.
Bibliografie
-
Reyes R, López-Castro R, Auclin E, et al. Impact of COVID-19 Pandemic in the Diagnosis and Prognosis of Lung Cancer. Presented at: 2020 World Conference on Lung Cancer Singapore; January 28-31; Virtual. Abstract 3700.
-
Guan WJ, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: A nationwide analysis. Eur Respir J. 2020;55(5):2000547.
-
Ji W, et al. Effect of underlying comorbidities on the infection and severity of COVID-19 in South Korea. J Korean Med Sci. 2020 Jun 29; 35(25):e237.
-
Garassino MC, Whisenant JG, Huang LC, Trama A, Torri V, Agustoni F, Baena J, Banna G, Berardi R, Bettini AC, Bria E, Brighenti M, Cadranel J, De Toma A, Chini C, Cortellini A, Felip E, Finocchiaro G, Garrido P, Genova C, Giusti R, Gregorc V, Grossi F, Grosso F, Intagliata S, La Verde N, Liu SV, Mazieres J, Mercadante E, Michielin O, Minuti G, Moro-Sibilot D, Pasello G, Passaro A, Scotti V, Solli P, Stroppa E, Tiseo M, Viscardi G, Voltolini L, Wu YL, Zai S, Pancaldi V, Dingemans AM, Van Meerbeeck J, Barlesi F, Wakelee H, Peters S, Horn L; TERAVOLT investigators. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020 Jul;21(7):914-922.
-
Aran V, De Marchi P, Zamboni M, Ferreira CG. Dealing with lung cancer in the COVID-19 scenario (A review). Mol Clin Oncol. 2021 Feb;14(2):27.
-
Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010 Mar 19;140(6):883-99.
-
https://datelazi.ro/
-
Verity R, et al. Estimates of the severity of coronavirus disease 2019: A model-based analysis. Lancet Infect Dis. 2020;20(6):669–677.
-
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018 Nov;68(6):394-424.
-
Burdett N, Desai J. New biomarkers for checkpoint inhibitor therapy. ESMO Open. 2020 Sep;5(Suppl 1):e000597.
-
Myers DJ, Wallen JM. Lung Adenocarcinoma. [Updated 2020 Jun 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK519578/
-
Maione P, Rossi A, Sacco PC, Bareschino MA, Schettino C, Ferrara ML, Falanga M, Ambrosio R, Gridelli C. Treating advanced non-small cell lung cancer in the elderly. Ther Adv Med Oncol. 2010 Jul;2(4):251-60.
-
Mayoral M, Castañer E, Gallardo X, Andreu M, Dalmau E, Garcia Y. Tumor pseudoprogression during nivolumab immunotherapy for lung cancer. Radiologia. 2019 Nov-Dec;61(6):498-505. English, Spanish. doi: 10.1016/j.rx.2019.05.004.
-
Onoi K, Chihara Y, Uchino J, Shimamoto T, Morimoto Y, Iwasaku M, Kaneko Y, Yamada T, Takayama K. Immune Checkpoint Inhibitors for Lung Cancer Treatment: A Review. J Clin Med. 2020 May 6;9(5):1362.
-
Seliger B. Basis of PD1/PD-L1 Therapies. J Clin Med. 2019 Dec 8;8(12):2168.
-
Ribas A, Hu-Lieskovan S. What does PD-L1 positive or negative mean? J Exp Med. 2016 Dec 12;213(13):2835-284.
-
You W, Shang B, Sun J, Liu X, Su L, Jiang S. Mechanistic insight of predictive biomarkers for antitumor PD 1/PD L1 blockade: A paradigm shift towards immunome evaluation (Review). Oncol Rep. 2020 Aug;44(2):424-437.
-
Yang F, Wang JF, Wang Y, Liu B, Molina JR. Comparative Analysis of Predictive Biomarkers for PD-1/PD-L1 Inhibitors in Cancers: Developments and Challenges. Cancers. 2022;14(1):109. https://doi.org/10.3390/cancers14010109.
-
Zouein J, Kesrouani C, Kourie HR. PD-L1 expression as a predictive biomarker for immune checkpoint inhibitors: between a dream and a nightmare. Immunotherapy. 2021 Aug;13(12):1053-1065.
Articole din ediţiile anterioare
Radiomica şi radiogenomica în managementul cancerului bronhopulmonar – o scurtă recenzie
Cancerul bronhopulmonar este principala cauză a mortalităţii prin cancer, diagnosticul tardiv fiind una dintre cauzele asociate cu decesul şi cu ...
Importanţa rebiopsierii pentru detectarea mutaţiei T790M în cancerul pulmonar fără celule mici – prezentare de caz
Lung cancer, the leading cause of mortality worldwide, is comprised in proportion of 80-85% of non-small cell lung carcinoma (NSCLC).
Real-world evidence with nivolumab in advanced non-small cell lung cancer – second line and beyond. Our experience in Romania following straightaway reimbursement of healthcare costs
Neoplasmul pulmonar reprezintă principala cauză a mortalităţii prin cancer la nivel mondial, ocupând locul al doilea în statisticile de incide...
Asocierea dintre inhibitorii de tirozin-kinază şi chimioterapie în cancerul pulmonar fără celule mici
Această recenzie încearcă să analizeze câteva studii şi metaanalize ale inhibitorilor de tirozin-kinază (TKI) în combinaţie cu chimioterapia. Une ...