The ”magic bullet” term was used for the first time by Paul Ehrlich in early 20th century, when he described antibodies that target both tumour cells and microbial agents. Immunotherapy in cancer therapy is a type of treatment discovered in the 1970s. To better understand the types of therapies and their indications and side effects, it requires a review of the immune reaction at the time that tumour cells appear and the mechanisms by which the cell manages to fool the immune response and develop malignant tumours, that metastasize and eventually destroy the host. Cancer immunotherapy involves the use of therapeutic modalities that lead to a manipulation of the immune system by using immune agents such as cytokines, vaccines, cell therapies and humoral, transfection agents. We intended to write a review article about immune reactions that take place into onset of cancer and new immunologic treatments developed in last years.
Imunoterapia în cancer: mecanismele imunologice şi rolul lor în terapie
Immunotherapy in cancer: mechanisms of immune response and their place in cancer treatments
First published: 04 aprilie 2017
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/OnHe.38.1.2017.549
Abstract
Rezumat
Termenul „glonț magic” a fost folosit pentru prima dată de Paul Ehrlich la începutul secolulului XX, când a descris anticorpii care ținteu atât celulele tumorale, cât și agenții microbieni. Imunoterapia în tratamentul cancerului este o modalitate de tratament descoperită în anii 1970. Pentru o mai bună înţelegere a tipurilor de terapii, a indicaţiilor, cât şi a efectelor adverse, este necesară înţelegerea mecanismului reacţiei imune a organismului în momentul apariţiei celulelor tumorale şi a mecanismelor prin care celula păcăleşte răspunsul imun şi dezvoltă o tumoare malignă care în cele din urmă metastazează şi distruge organismul-gazdă. Imunoterapia cancerului implică folosirea de modalităţi care duc la manipularea sistemului imun folosind agenţi imuni precum citokinele, vaccinurile, terapiile celulare şi umorale, agenţi de transfer. În acest articol prezentăm reacţiile imune care au loc în momentul apariţiei celulei tumorale şi noile tratamente imunologice dezvoltate în ultimii ani.
Immunotherapy in cancer therapy is a type of treatment discovered in the 1970s, with the onset of bladder cancer therapy with BCG and IFN therapy in malignant melanoma(1). There were discovered various immune therapies such as IL 2 cytokine used in solide tumors like melanoma. A period of decline of these therapies followed, with powerful side effects and minor results in treatments. Along with studying the mechanisms of immune cells involved in the immune response mediators that cause stimulation or inhibition of the immune response, came the development of new therapies. To better understand the types of therapies and their indications and side effects, it requires a review of the immune reaction at the time that tumour cells appear and the mechanisms by which the cell manages to fool the immune response and develop malignant tumours, that metastasize and eventually destroy the host.
Cancer immunotherapy involves the use of therapeutic modalities that lead to a manipulation of the immune system by using immune agents such as cytokines, vaccines, cell therapies and humoral, transfection agents(2).
Tumour cells differ from normal cells, by expressing the antigen and biologic behaviour. Genetic instability is the main generator of the cell cancerous tumour-specific antigens. Recognizing these tumour-specific antigens on the cell surface is the centerpiece of immune stimulation(3).
Tumour cells express cell surface proteins mutate, fusion proteins or protein aberrantly self expressed, so that the immune system should recognize them. Immunotherapy uses several methods to manipulate the antitumor immune system of the passive immunization with monoclonal antibodies or the induction of systemic cytokine administration of the adjuvant to the tumour microenvironment(4).
Antigens are harmful substances, such as parts from bacteria, viruses, fungi or parasites and they can also appear on cancer cells. Antibodies are proteins that bind antigens (and help, for example, to fight infection).
Antibodies are a key component of the adaptive immune response, playing a central role both in the recognition of foreign antigens and in the stimulation of an immune response to them. It is not surprising, therefore, that many immunotherapeutic approaches involve the use of antibodies.
Monoclonal antibodies are made in a laboratory and are directed against a specific protein in the cancer cells, and they do not affect the cells that do not have that protein. When they are given to patients, they act like the antibodies the body produces naturally(5).
Types of monoclonal antibodies
Two types of monoclonal antibodies are used in cancer treatments:- Naked monoclonal antibodies are antibodies without modification.
- Conjugated monoclonal antibodies are joined to another molecule, which is either toxic to cells, or radioactive. The toxic chemicals are those typically used as chemotherapy drugs, but other toxins can be used. The antibody binds to specific antigens on cancer cell surfaces, directing the therapy to the tumour. Radioactive compound-linked antibodies are referred to as radiolabelled. If the antibodies are labelled with chemotherapy or toxins, they are known as chemolabelled or immunotoxins, respectively.
- It stimulates the immune system to destroy the malignant cell (Figure 2). The immune system doesn’t always recognize cancer cells as being harmful, but monoclonal antibody can mark cancer cells by attaching to specific parts of cancer cells not found on healthy cells (tumour specific antigens). This makes it easier for the immune system to find and destroy these cells. In present, the monoclonal antibodies that target the PD-1 protein, which are intensely studied, are a good example. PD-1 keeps the immune system from recognizing that a cell is cancerous, so drugs that block PD-1 - a new class of drugs - allow the immune system to identify and eliminate the cancer(6).
- Prevent cancer cells from growing rapidly. Growth factors tell cells to grow by attaching to receptors on the surface of cells. The receptor they attach to is called a growth factor receptor. Cancer cells grow faster than normal cells because they can make extra copies of the growth factor receptor. Monoclonal antibodies can block these receptors and prevent the growth signal from getting through.
- Deliver different treatments - radiotherapy - directly to cancer cells. Radio-immunotherapy uses monoclonal antibodies to deliver radiation directly to cancer cells by attaching radioactive molecules to monoclonal antibodies in a laboratory. They deliver low doses of radiation specifically to the tumour while leaving healthy cells alone - for example, ibritumomab tiuxetan (Zevalin) and tositumomab (Bexxar).
- Diagnose cancer (type and localisation). The pathologist may use monoclonal antibodies to determine the type of cancer a person may have by analyzing the sample of tissue removed during biopsy. Monoclonal antibodies carrying radioactive particles may also help diagnose certain cancers, such as colorectal, ovarian, and prostate cancers.
- Deliver drugs directly to cancer cells. This causes the cancer cell to die without damaging other healthy cells. Some monoclonal antibodies carry other cancer drugs directly to cancer cells, and once the monoclonal antibody attaches to the cancer cell, the treatment it is carrying enters the cell - for example, Brentuximab vedotin (Adcetris) for Hodgkin and non-Hodgkin lymphoma, trastuzumab emtansine or TDM-1 (Kadcyla) for HER2-positive breast cancer(7,15).
Chimeric antibodies were the first attempt to reduce the immunogenicity of these antibodies. They are murine antibodies with a specific part of the antibody replaced with the corresponding human counterpart, known as the constant region.
Humanized antibodies are almost completely human; only the complementarity determining regions of the variable regions are derived from murine antibodies. Human antibodies have completely human DNA.
Antibodies are formed of a binding region (Fab) and the Fc region that can be detected by immune cells via their Fc surface receptors. Fc receptors are found on many immune system cells, including natural killer cells.
Cell death mechanisms
Antibody-dependent cell-mediated cytotoxicity (ADCC)Antibody-dependent cell-mediated cytotoxicity (ADCC) is a mechanism of attack by the immune system that requires antibodies to bind to target cell surfaces. When natural killer (NK) cells encounter antibody-coated cells interact with their Fc receptors, leading to the release of perforin and granzyme B.
Complement
The complement system includes blood proteins that can cause cell death after an antibody binds to the cell surface (this is the classical complement pathway, among the ways of complement activation). The system can be activated with therapeutic antibodies in cancer. The system can be triggered if the antibody is chimeric, humanized or human; as long as it contains the IgG1 Fc region. The complement can lead to cell death by activation of the membrane attack complex.
Immune response to tumours is divided into innate and adaptive immune response. Into innate immune response are implicated NK cells and macrophages, and into adaptive immune response are implicated T cells and antibodies (aforementioned).
The first few transformed cells are detected by NK cells through their encounter with specific ligants on tumor cells. These leads to the destruction of some transformed cells and the uptake and processing of their fragments by macrophages and dendritic cells. These macrophages and dendritic cells are activated to secrete many inflammatory cytokines and present tumour cells - derived molecules to T&B cells. Activation of T&B cells leads to the production of cytokines that promote activation of innate immunity and support the expansion and production of tumour specific T cells and antibodies. The adaptive immune system leads to the elimination of resting tumour cells and to the generation of immune memory to tumour components.
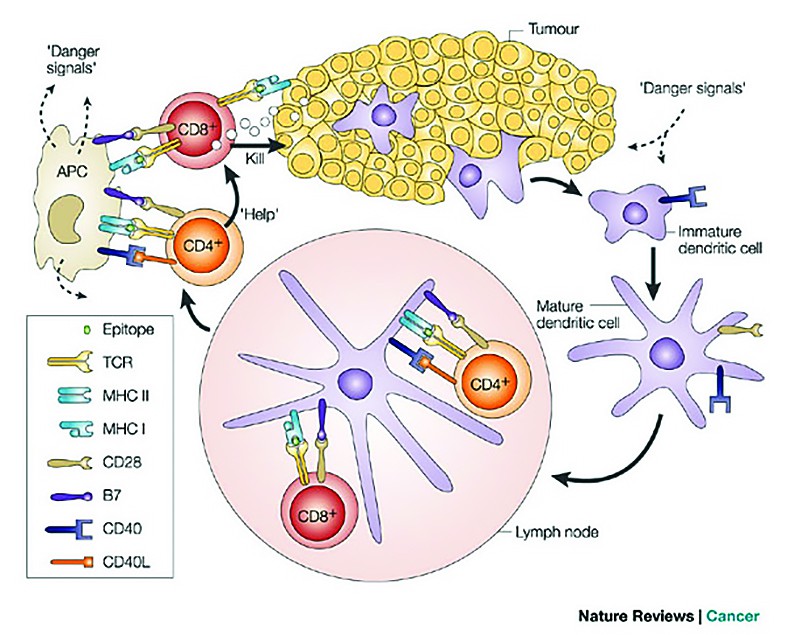
Cellular effectors that mediate immunity are (Figure 1):
1. Cytotoxic T lymphocytes - which has a protective role against virus/tumour antigen-associated neoplasms (for example, HPV, EBV)(8).2. Natural killer cells (NK) - which are capable of destroying tumour cells without prior sensitization (the first line of defense against tumours). After activation with IL2, NK lyse a variety of tumours even if they appear to be non-immunogenic to T cells.
3. Macrophages - which when activated exhibit selective cytotoxicity against tumour cells. T cells, NK cells and macrophages collaborate in anti-tumour reactivity. INF γ secreted by T and NK cells activates macrophages, killing by production of reactive oxygen or secretion of TNF α.
4. Humoral mechanisms and activation of complement
Dendritic cells
These cells take in available information about threats throughout the body regrouped at headquarters (secondary lymphoid organs), and alert other cells.
They present antigens to lymphocytes, which activates them, priming them to kill other cells that present the antigen. The only approved cellular therapy for cancer is Sipuleucell-T(9).
B cells
When activated, they turn into plasmocytes that can produce thousands of highly-targeted antibodies(10).
Regulatory T cells provide the checks that help insure that immune responses don’t create collateral damage on healthy cells.
CD4 helper T cells
These cells provide orders and support other cells, including B cells and CD8 plus killer T cells(11).
CD8 killer T cells
CD8 T cells are the killers of the immune system. They look for and destroy cells that have pro-aberrant proteins expressed onto the surfaces.
Cancer is an immunological disorder
Immunotherapy is a common denominator that can activate immune responses against mutant proteins present on tumour cells. The adaptive immune system can keep up with tumour evolution.
Tree phases are necessary to develop cancer: first is represented by elimination, the second is represented by equilibrium and the third is escape. Elimination means that immune system detects and destroys cancer cells as they develop, eliminating them before they form tumours.
Equilibrium means that immune system has destroyed some cancer cells, while others less visible by the immune system remain and the two remain into a state of equilibrium.
Escape means that the remaining cancer cells overcome the immune system and start to multiply, forming clinical detectable tumours.
At this stage, the immune system is incapable to control cancer growth on its own.
Initially, most of the escaped from the immune-surveillance was ascribed to changes in the tumours cells themselves (loss of tumour antigens, loss of human leukocytes antigen molecules, loss of sensitivity to complement, or T cells or natural killer cell leasys), making them a poor target of an immune attack.
However, it has become clear that the suppression comes from the ability of tumours to subvert normal immune regulations to their advantage.
The main limiting factor is tumour immune tolerance and immunology energy that are responsible of:
Tumour cells by:
1. The production of immunosuppressive cytokines (TGFβ, IL10, VEGF).
2. Inhibition of MHC class I expression (major complex of histocompatibility).
3. Decreased expression of TAA (tumour antigen associated).
4. Inhibition of apoptosis: overexpression of anti-apoptotic gene products such as Bcl-2 and v-Rel, overexpression of cFlip that inhibits the caspase.
5. Stimulating regulatory LT (T lymphocytes) - inhibit - are grown in peripheral blood and peritumoral.
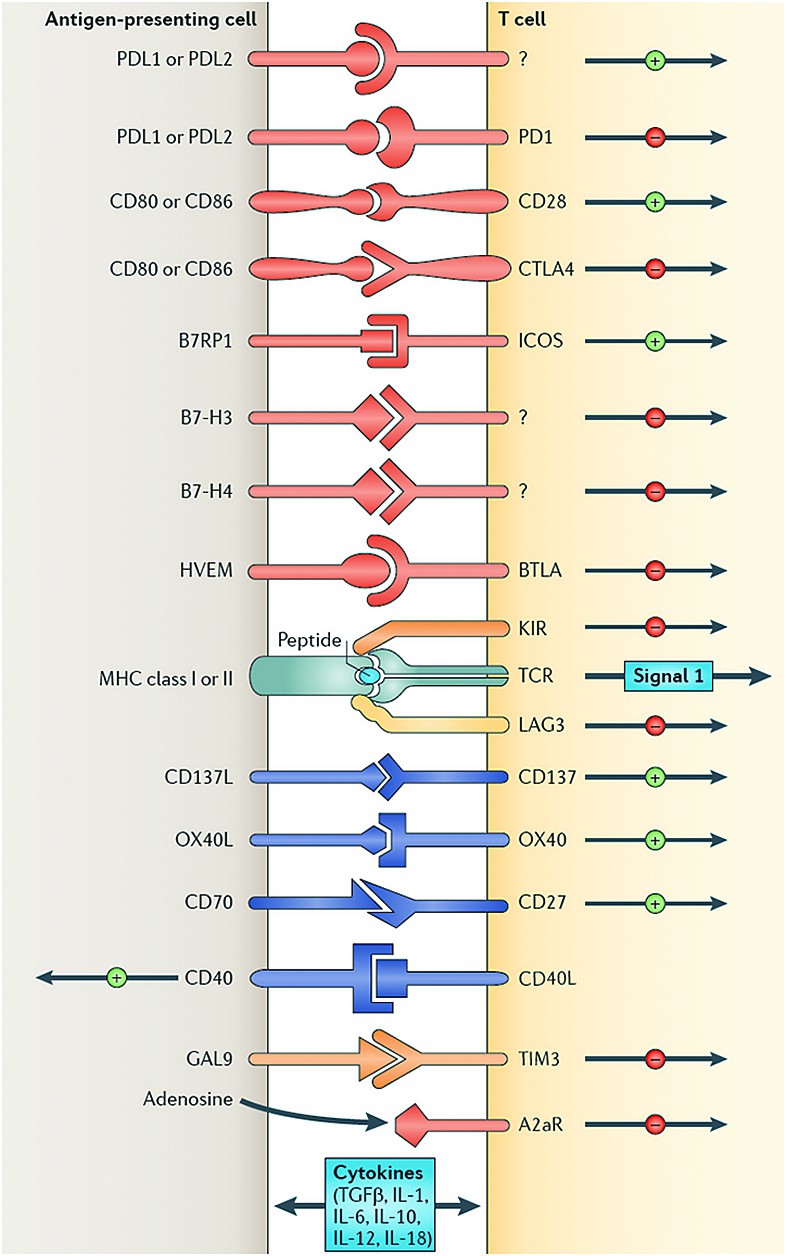
6. Absence costimulatory - costimulatory molecules: in family B7 (CD 80, CD 86) CD 40L ligand, intercellular adhesion molecules ICAM.
Tumor microenvironment by:
1. Prostaglandins
2. Reactive Oxygen Species
3. Nitric Oxide(12).
Immunotherapy of cancer (Figure 2)
1. Stimulates host’s anti-tumor response by increasing effector cell number (like DC based vaccines) and production of soluble mediators (like increase tumor cell immunogenicity).
2. Decreases host’s suppressor mechanisms by inducing tumour killing environmentand by modulating immune checkpoints.
Cancer immunotherapy - treatments that enhance the innate powers of the immune system to fight cancer - represents the most promising new cancer treatment approach since the development of the first chemotherapies in the late 1940s.
Antitumour immunotherapy involves: active immunization by cancer vaccines, stimulating non-specific immune reactions by stimulating effector cells and regulating cell inhibition, passive transfer of activated immune cells which have antitumour activity. Antitumour immunotherapy strategies: use to boost immunity cytokines, monoclonal antibodies, cancer vaccines, adaptive T-cell therapy(13).
Nonspecific therapy: immune cytokines
IL2 activates LT proliferation, increases LT killer activity, activates LAK (activated killer lymphocytes), proliferation and Ig secretion by LB, and increases the secretion of IL-1, IL6, TNF, IFN γ.IFN has antiangiogenic effect, it stimulates apoptosis, and increases the expression of MHC and TAA(14,15).
Monoclonal antibody therapy has antitumour effects in the signaling by receptor binding, antibody-dependent cellular cytotoxicity, complement dependent cytotoxicity.
Monoclonal antibodies unconjugated: e.g. Rituximab- monoclonal antibody anti CD used in nonHodgkin lymphoma:, Alemtuzumab- antibody anti CD 52 used in CLL; Ofatumumab- anti CD 20 LB LLC refractory, Epratuzumab antibody anti CD22 – used in follicular lymphoma, Trastuzumab- anti Her receptor- used in Her 2 positive breast cancer, gastric cancer, Pertuzumab-anti HER2 receptor in Her2 positive breast cancer, Bevacizumab-anti VEGF used in colon cancer, renal cancer, NSCLC; Cetuximab- anti EGFR- RASwt- used in colon cancer, head and neck cancer, Panitumumab - anti EGFR- colorectal cancer, Denosumab RANK ligand inhibitor- used in bone metastases(7).
Immunomodulators specific: for example, Ipilimumab - CTLA 4 inhibitors act by inhibiting the immune system associated antigen 4 LTc, anti PD1- Nivolumab - melanoma, NSCLC, renal cancer, tremelimumab, Pembrolizumab - melanoma, Pidilizumab, anti PD-L1.
Ipilimumab is a type of immune drug known as a checkpoint inhibitor. These treatments work by targeting molecules that serve as checks and balances in the regulation of immune responses. By blocking inhibitory molecules, these treatments are designed to unleash or enhance pre-existing anti-cancer immune responses.
Conjugated monoclonal antibodies: for example, Gemtuzumab - antibiotic anti CD3 conjugated antitumoral - LAM Denileukindifitox- fusion protein IL2 /toxin difterica - cutaneous T lymphomas, murine Ibritumomabtixetan - with non-Hodgkin Yritriumradioactiv - malignant lymphoma (NHL), Tositumomab - murine anti CD20 bound iodine radioactive - NHL, Trast-DM1 TDM1(16).
Antitumoral vaccines - made by the whole tumour cell, genetically modified tumour cells or peptide anti PD1 - without clinical efficacy(13).
Passive immune therapy: adoptive cell transfer
TIL (tumour infiltrating lymphocytes), introduced directly into the tumour T cells after ex vivo culture, in the presence of malignant melanoma IL2(17).
The importance of immune therapy can be easily described in practice, especially in malignant melanoma. From the preventive vaccine for cervical cancer to the first therapy ever proven to extend the lives of patients with metastatic melanoma, immunology has already led to major treatment breakthroughs for a number of cancers(18).
Bibliografie
2. Davies, D. H. Immune System. Encyclopedia of Life Sciences, www.els.net. Nature Publishing Group (2001).
3. Heath W.R. & Scott H.S. Immunology: Education and promiscuity, Nature (2002) 420:468-9.
4. Evan, G.I. & Vousden, K.H.. Proliferation, cell cycle and apoptosis in cancer. Nature (2001), 411:342-348. 38.
5. Halim, N.S. Monoclonal Antibodies: A 25-Year Roller Coaster Ride. The Scientist (2000) 14, No. 4, page 16.
6. James, K. Immune Responses: Primary and Secondary. Encyclopedia of Life Sciences (2001), www.els.net. Nature Publishing Group, DOI: 10.1002/9780470015902.a0000947.pub2
7. Coty, C. Monoclonal antibodies look to a bright future. Drug Discover & Development Vol 5, No6, 45-49 (2002).
8. Martindale D. T cell triumph: immunotherapy may have finally turned a corner. Scientific American, February 2003, 288, 18-19, doi:10.1038/scientificamerican0203-18.
9. Banchereau, J & Steinman, R.M. Dendritic cells and the control of immunity. Nature (1998), Vol 392, 245-252.
10. Jager E. et al. Clinical cancer vaccine trials. Current Opinion in Immunology. (2002) Vol 14(2):178-82.
11. Boes, M. et al. (2002) T-cell engagement of dendritic cells rapidly rearranges MHC class II transport. Nature 418:983-8.
12. Kiberstis P. & Marx J. The Unstable Path to Cancer. Science July (2002), 26; 297: 543.
13. Rosenburg SA, Yang JC, Rstifo NP. Cancer immunotherapy: moving beyond current vaccine, Nat Med, 2004 septembrie; 10 (9): 909-915.
14. Clark WR. At War Within: The Double-Edged Sword of Immunity. Oxford University Press, Oxford, 1995.
15. Kaplan D.H. et al. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. PNAS (1998) 23; 95(13):7556-61.
16. European Medicine Agency: www.ema.europa.eu – accesed nov 2015
17. Rosenburg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes, Science. 1986 Sep 19; 233(4770):1318-21.
18. Davies D.H. Immune System. Encyclopedia of Life Sciences, www.els.net. Nature Publishing Group, 2001.
Articole din ediţiile anterioare
Imunoterapia în cancerul pancreatic - un pas înainte în viitor
De zeci de ani a existat o permanentă preocupare pentru a descoperi relația între tumoră și sistemul imun. Ultimele descoperiri au permis folosirea...
Second primary breast cancer during PARP inhibitor treatment of somatic BRCA mutant ovarian cancer – a case report
Ovarian cancer is the fifth cause of death by cancer in the female population from Romania(1). Mutations in the BRCA1 (breast cancer gene 1) and BR...
Adenocarcinom mucinos pulmonar primitiv la o femeie nefumătoare
Adenocarcinoamele sunt cele mai frecvente tipuri histologice de cancer pulmonar. Dintre ele, adenocarcinomul mucinos pulmonar primitiv (fostu...
Importanţa factorului neurotrofic derivat din creier în menţinerea sănătăţii cerebrale în timpul şi după tratamentele oncologice
Datorită numeroaselor descoperiri ştiinţifice din ultimele decade, supravieţuirea pacienţilor cu cancer a crescut semnificativ, astfel că 70% dintr...