Follicular lymphoma (FL) is the most common indolent non-Hodgkin lymphoma. The clinical behavior is heterogeneous, with many clinical scenarios, including long-term stable disease, disease progression, histological transformation, and early or tardive relapses after treatment. The most important predictors for disease aggressivity are the Follicular Lymphoma International Prognostic Index, the histological subtype, the proliferation index, the Ann Arbor staging, the tumoral burden, the relapse time and the histological transformation. The treatment options in FL are numerous, ranging from watchful waiting to radiation therapy, aggressive chemoimmunotherapy, and even stem cell transplantation. The treatment is chosen based on disease (stage, grade) and patient’s characteristics (age, comorbidities, preference). We present the case of a 69-year-old patient diagnosed with grade 3a follicular lymphoma, treated with chemoimmunotherapy and radiation therapy, with an aggressive evolution. Although she obtained a good response after therapy, the disease relapsed rapidly. The management was complicated even more when the patient developed an indolent SARS-CoV-2 infection.
Limfom non-Hodgkin cu evoluţie agresivă – prezentare de caz
Aggressive behavior of indolent non-Hodgkin lymphoma – case report
First published: 24 aprilie 2021
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/OnHe.55.2.2021.5019
Abstract
Rezumat
Limfomul folicular este cel mai frecvent limfom non-Hodgkin indolent. Evoluţia clinică este heterogenă, cu multe scenarii clinice, incluzând stabilitatea bolii pe termen lung, progresia bolii, transformarea histologică sau recidive precoce ori tardive după tratament. Cei mai importanţi factori pentru prezicerea agresivităţii bolii sunt scorul Follicular Lymphoma International Prognostic Index, subtipul histologic, indicele de proliferare, stadializarea Ann Arbor, încărcătura tumorală, timpul până la recidivă şi transformarea histologică. Opţiunile de tratament în limfomul folicular sunt numeroase, de la monitorizare atentă la radioterapie, chimioterapie agresivă şi chiar transplant de celule stem. Alegerea tratamentului se bazează pe caracteristicile bolii (stadiu, grad) şi ale pacientului (vârstă, comorbidităţi, preferinţă). Prezentăm cazul unei paciente în vârstă de 69 de ani, diagnosticată cu limfom folicular de gradul 3a, tratată cu imunochimioterapie şi radioterapie, cu o evoluţie agresivă. Deşi a obţinut un răspuns bun la terapie, boala a recidivat rapid. Managementul a fost complicat de contractarea unei infecţii indolente cu SARS-CoV-2.
Introduction
Follicular lymphoma (FL) is a heterogeneous lymphoproliferative disease, characterized by tumors derived from germinal center cells, namely centroblasts and centrocytes. Worldwide, FL is the most common type of indolent non-Hodgkin lymphoma, accounting for approximately 60% of low-grade lymphomas, as reported in literature(1).
The clinical evolution is influenced by many factors, the most significant being the Follicular Lymphoma International Prognostic Index (FLIPI) and the histological grading. Other major predictors of aggressivity are the short duration of remission after the initial treatment, disease progression, and histological transformation(4-6).
According to the World Health Organization, FL is divided into three morphological categories, depending on the proportion of centroblasts; grade 3 is further subdivided into types a and b(2). The clinical evolution is connected to morphological classification. Grades 1-2 and 3a are considered indolent, while grade 3b is considered an intermediate-grade lymphoma, with higher mortality(1,3).
FLIPI was developed in order to improve the treatment choice. It uses five independent prognostic factors, namely age, Ann Arbor stage, number of involved nodal sites, LDH and hemoglobin level(6). The histological grade, considered one of the most important factors in choosing the treatment, is not comprised in this score(7).
Although most patients present in an advanced stage, the median survival is high and is constantly improving due to the advances in management(1).
The therapeutic strategies for FL are numerous, and should be individualized based on patient and disease characteristics, mainly grade, stage, FLIPI, age and comorbidities. The treatment options include watchful waiting, radiotherapy, anti-CD20 monoclonal antibodies, chemotherapy, lenalidomide, PI3K inhibitors, EZH2 inhibitor and even allogeneic hematopoietic cell transplantation in selected cases(8). The National Comprehensive Cancer Network guidelines recommend that grade 3b FL should be treated as diffuse large B-cell lymphoma. The treatment of grade 3a is debatable, and should be managed as FL or as DLBCL, depending on patient characteristics(8).
Case presentation
We present the case of a 69-year-old female patient who was admitted to our clinic for an infraorbital tumor and retromandibular adenopathy. Her medical history was positive only for hepatitis B, developed and remitted 20 years before presentation. The clinical examination did not reveal other modifications.
The biological findings were unremarkable, as shown in Table 1.
Imagistic examinations were performed. The cervical magnetic resonance imagistic (MRI) confirmed the presence of the infraorbital tumor (maximum diameter of 38 mm), of retromandibular adenopathy, and also small cervical adenopathies. The computed tomography (CT) showed no other abnormalities. Biopsies were taken from the tumor and mandibular adenopathy; the histopathological and immunohistochemical studies revealed follicular lymphoma grade 3a, with a high proliferation index (Ki-67 of 40%).
The FLIPI revealed a low-risk disease, with only one adverse factor, namely age over 60 years old. The bone marrow biopsy showed no malignant infiltrate.
These findings support the diagnosis of follicular lymphoma, grade 3a, stage IIA.
The patient was treated in according to the protocol with R-CVP (rituximab, cyclophosphamide, vincristine and prednisone). After six cycles, the CT scan showed the complete regression of infraorbital tumor and adenopathies. The treatment was continued with rituximab maintenance every two months.
After five administrations of rituximab, at 14 months since diagnosis, the patient presented with progressive back pain. An MRI was performed; it showed a tumoral mass (114/41/60 mm) located in the paravertebral muscles, infiltrating the adjacent vertebrae, associating acquired stenosis in T10-L2 segments and epiduritis. The biopsy confirmed the diagnosis of follicular lymphoma grade 3a, with a Ki-67 of 75%. For a complete imagistic picture, a CT scan was performed; it showed no other involved sites. The patient was referred to a neurosurgery clinic; it was decided that the patient was not eligible for surgery.
Therefore, systemic chemotherapy was considered the best option. The patient underwent eight cycles of R-CHOP regimen (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone). The patient gradually became asymptomatic. Repeated CT scans showed tumoral regression, up to 38/28 mm after eight cycles. The whole-body PET-CT confirmed abnormal fluorodeoxyglucose (FDG) activity at the tumoral site, with a maximum standardized uptake value (SUV) of 8.97. Consequently, we completed the treatment with intensity-modulated radiation therapy, 40 Gy in 20 fractions. The treatment was well tolerated, with only mild, manageable hematologic and cutaneous adverse reactions.
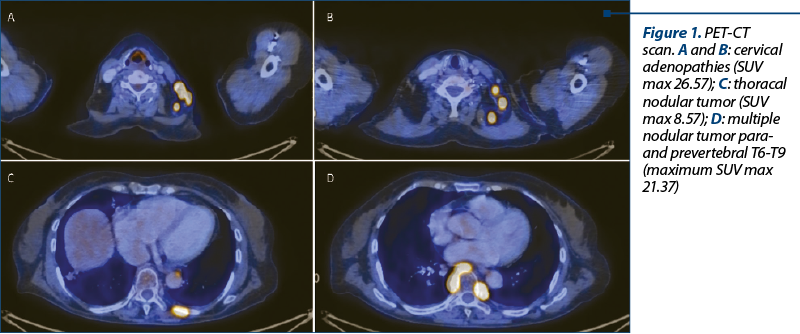
A CT scan was performed after radiotherapy; it showed a favorable evolution, although the paravertebral tumor was still visible. A PET-CT was scheduled. Meanwhile, the patient developed back pain and cervical adenopathy, but did not present for a consultation. The PET-CT depicted the disappearance of the previously mentioned paravertebral tumor, but instead it described multiple adenopathies and tumoral masses, having a high SUV max of 26.57 (Figure 1).
An excisional biopsy established the same diagnosis – follicular lymphoma grade 3a, a second early relapse in 29 months of evolution, at only two months after completing radiotherapy. Thus, the patient received treatment according to the international guidelines, with obinutuzumab and bendamustine. The treatment was well tolerated, and the patient also observed a slight decrease in back pain.
On day 15 of the first cycle, a routine RT-PCR for SARS-CoV-2 was performed. The test came out positive, although the patient was completely asymptomatic at that time. The clinical exam was unremarkable; the complete blood count showed only lymphopenia (0.3 x 109/L).
The patient refused hospitalization, and she remained isolated at home for 14 days. During this time, she was paucisymptomatic, describing only fatigue and low fever (<38°C).
The biological findings after isolation revealed pancytopenia and a significant inflammatory syndrome (Table 2).
Discussion
The clinical evolution on Fl is highly variable, therefore it is important to be able to predict outcomes and to manage them accordingly. The most important variables that influence or predict the clinical behavior are tumoral burden, histology, stage, intratumoral and microenvironmental markers, proliferation index and FLIPI. Early relapse after initial treatment, disease progression and histological transformations are considered markers for an aggressive disease(4-6,9,10).
We presented a FL patient who presented with extranodal tumor, retromandibular and cervical adenopathies. The patient had grade 3a FL and a FLIPI score of 1, the only negative prognostic factor being the age over 65 years old. However, there are controversies regarding the use of the FLIPI score in grade 3 FL. While grades 1 and 2 may benefit from this score, grade 3 FL should receive more aggressive treatment, namely an anthracycline-containing regimen, independent of the FLIPI score(7).
During a disease evolution of only 29 months, the patients suffered two early relapses. Each time, we evaluated a potential histological transformation, but the results showed the same grade 3a FL.
The first relapse occurred during rituximab maintenance, in a different site than the initial one. The predictors for an aggressive evolution present at diagnosis were grade 3a, although controversial, and a high proliferative index (Ki-67 40%). The proliferation index was even higher at the first relapse, respectively of 75%. Ki-67 usually correlates with the histological grading, and it is also a useful prognostic marker. Kawaguchi et al. reported a discordant proliferation index in 17% of FL cases, and it was associated with short overall survival(9).
The second relapse occurred two months after finalizing radiation therapy and after 29 months since diagnosis. The factors that predicted an aggressive evolution were a previously early relapse with a high proliferation index and a high tumoral burden (bulky disease). The PET/CT scan that documented the second relapse showed multiple tumors with a high SUV, the highest being 26.57. Studies show that the FDG is correlated to the aggressivity of disease. An SUV max >10 is associated with aggressive histology in 81% of cases(11). Therefore, we had a high suspicion of histological transformation, although the biopsy did not confirm it.
The management was further complicated by an indolent SARS-CoV-2 infection, that occurred during the first cycle of obinutuzumab and bendamustine.
Patients diagnosed with lymphoid malignancies and treated with anti-CD20 monoclonal antibodies have a higher COVID-19-related mortality. Also, it seemed that the patients treated with anti-CD20 antibodies may not develop IgG antibodies and may have a delayed clearance of the virus(12).
The treatment for indolent lymphoma during the COVID-19 pandemic is not standardized. The European Society for Medical Oncology and the European Hematology Association Guidelines recommend evaluating risks and benefits when deciding to treat an indolent lymphoma. In the context of SARS-CoV-2 infection, the recommendation is delaying the treatment until viral clearance, if possible(13).
Conclusions
The presented case highlights the heterogeneous behavior of FL. Although our patient presented with low-grade indolent lymphoma, the clinical evolution was an aggressive one, with two early relapses in only 29 months of evolution. The SARS-CoV-2 infection complicated even more the management of the patient.
Bibliografie
-
Greer JP, Rodgers GM, Glader B, et al. Wintrobe’s Clinical Hematology: Fourteenth edition. Philadelphia, PA. Wolters Kluwer, 1069-1095, 2019.
-
Harris NL, Swerdlow SH, Jaffe ES, et al. Follicular lymphoma. In: WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. Thiele&JW Vardiman, IARC, Lyon, 2008.
-
Wahlin BE, Yri OE, Kimby E, et al. Clinical significance of the WHO grades of follicular lymphoma in a population-based cohort of 505 patients with long follow-up times. Br J Haematol. 2012;156(2):225-33.
-
Casulo C, Byrtek M, Dawson KL, et al. Early Relapse of Follicular Lymphoma After Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone Defines Patients at High Risk for Death: An Analysis From the National LymphoCare Study. J Clin Oncol. 2015;33(23):2516-22.
-
Kridel R, Chan FC, Mottok A, et al. Histological Transformation and Progression in Follicular Lymphoma: A Clonal Evolution Study. PLoS Med. 2016;13(12):e1002197.
-
Solal-Céligny P, Roy P, Colombat P, et al. Follicular lymphoma international prognostic index. Blood. 2004;104(5):1258-65.
-
Ghielmini M, Mora O. Does the FLIPI apply to grade 3 follicular lymphoma? Blood. 2005;105(12):4892; author reply 4892-3.
-
Mary Dwyer N, Hema Sundar M, Fakhri B, et al. NCCN Guidelines Version 4.2021 B-Cell Lymphomas Continue NCCN Guidelines Panel Disclosures. [accessed on 11 May 2021]; Available online: https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf
-
Kawaguchi Y, Shiozawa E, Shimada S, et al. Ki-67 expression of immunohistochemistry using computerized image analysis is a useful prognostic marker in follicular lymphomas. Int J Clin Exp Pathol. 2018;11(7):3366-3374.
-
Casulo C. Prognostic factors in follicular lymphoma: new tools to personalize risk. Hematology. American Society of Hematology Education Program. 2016;2016(1):269–276.
-
Smith SD, Redman M, Dunleavy K. FDG PET-CT in follicular lymphoma: a case-based evidence review. Blood. 2015;125(7):1078-1082.
-
Lopez CA, Chitty D, Chi J, et al. Outcomes in Patients with Hematological Malignancies Receiving Anti-CD20 Therapy in the Setting of COVID-19 Infection. Blood. 2020;136(1):8–9.
-
ESMO-EHA clinical practice guideline for management of malignant lymphoma – recommendations for the second phase of the COVID-19 pandemic: indolent B-NHL (follicular and marginal zone lymphoma, Waldenstrom’s macroglobulinemia). [accessed on 11 May 2021]; Available online: https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic