Adenopatiile cervicale la copil – mereu o provocare
Cervical lymphadenopathies in children – always a challenge
Abstract
Cervical lymphadenopathy is a real challenge for the pediatrician. It is a possible symptom of infectious, but also malignant diseases. Cervical lymphadenopathy may be congenital, neoplastic, or most commonly inflammatory. Some of the infectious causes include atypical mycobacteria, tuberculosis, Bartonella henselae, fungal, parasitic or opportunistic infections. Neoplastic causes include primary tumors, but neoplasia leading to cervical lymphadenopathy is much more common and includes lymphoma and metastatic disease. The diagnosis is based on a detailed history and a correct clinical examination. Sonography also plays an important role in the differential diagnosis of lymphadenopathy, but sometimes only the serological tests confirm the clinical and ultrasound suspicion. If a condition persists or malignancy is suspected, a histological examination is required, the gold standard being the complete dissection of the affected lymph node.Keywords
lymphadenopathycat-scratch diseaseBartonella henselaeRezumat
Limfadenopatia cervicală este o adevărată provocare pentru medicul pediatru, fiind un posibil simptom al bolilor infecţioase, dar şi maligne. Masele adenopatice cervicale pot fi congenitale, neoplazice sau inflamatorii, ultimele fiind etiologia cea mai frecventă. Unele dintre cauzele infecţioase includ micobacteriile atipice, tuberculoza, Bartonella henselae, infecţiile fungice, parazitare sau oportuniste. Cauzele neoplazice includ tumorile primare, dar neoplazia care conduce la limfadenopatie cervicală este mult mai frecventă şi include limfomul şi bolile metastatice. Diagnosticul se bazează pe o anamneză detaliată şi pe un examen clinic efectuat corect. Sonografia joacă, de asemenea, un rol important în diagnosticul diferenţial al adenopatiilor, însă uneori doar testele serologice confirmă suspiciunea clinică şi ecografică. Dacă o afecţiune persistă sau se suspectează malignitatea, se impune examinarea histologică, standardul de aur fiind reprezentat de disecţia completă a ganglionului afectat.Cuvinte Cheie
adenopatiiboala zgârieturii de pisicăBartonella henselaeCase presentation
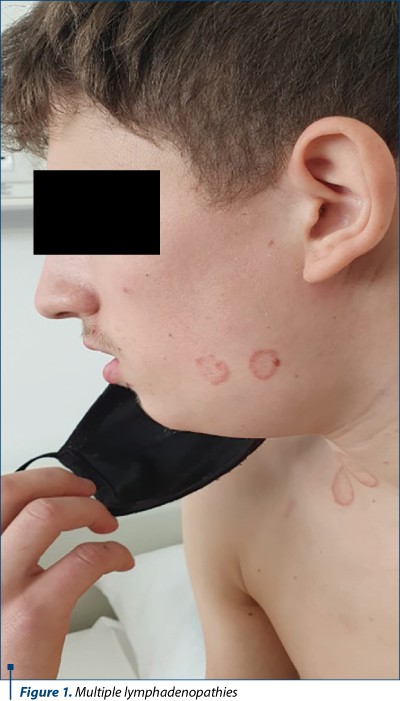
The patient D.R.G., teenager, 15 years old, from a rural area, hospitalized for the first time in the pediatric clinic, was admitted for multiple significant lymphadenopathies localized in the cervical, submandibular, submental (Figure 1) and axillary regions. The onset was four days before. Lymph nodes were painful, mobile, with a tendency to confluence, associated with skin erythema, fever, sore throat, painful swallowing and intermittent headache. He also associated skin circinate lesions. He had been scratched in the cervical region by a 6-month-old cat a week before the onset of lymphadenopathy.
The family history was insignificant, without contact with persons diagnosed with tuberculosis or other infectious diseases. His family had animals (cat, dog, cow, horse).
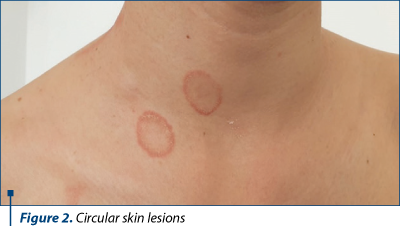
At the time of admission, the patient had an affected general condition, age-appropriate anthropometric parameters, circular skin lesions with well-defined edges and a diameter of about 2-3 centimeters located on the neck, limbs and chest (Figure 2), multiple lymphadenopathies located subangulomandibular, submental and cervical, with a diameter of 1-4 centimeters, with a tendency to confluence, mobile, firm in consistency, extremely painful, and axillary, with approximately 1 centimeter in size, associating headache, without meningeal irritation signs.
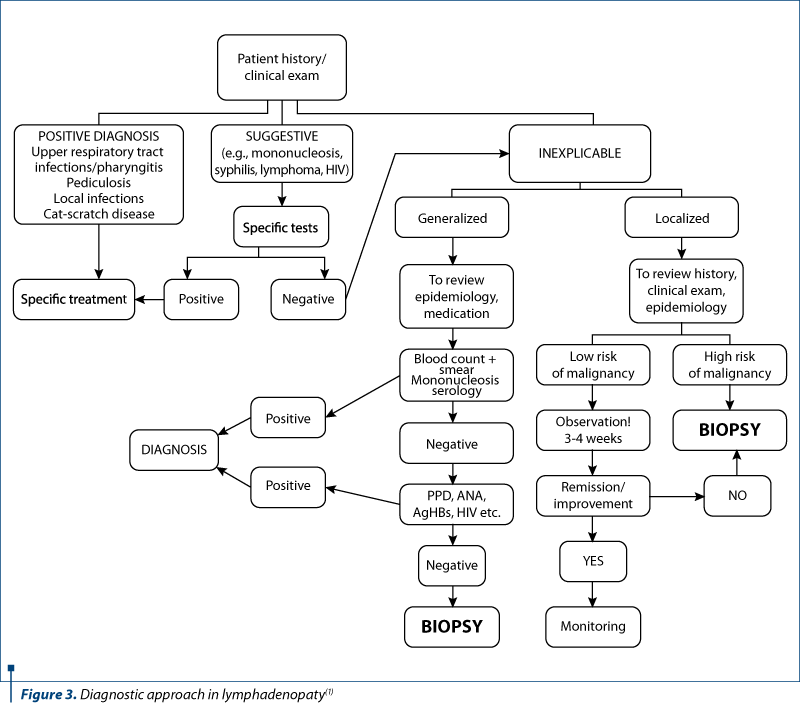
In front of a cervical lymphadenopathy, a diagnostic algorithm is required (Figure 3).
According to this protocol, the following investigations have been done: the inflammatory syndrome – positive values (ESR=28 mm/1 h, CRP=23.1 mg/l), complete blood count – normal values (WBC=10,780/µL, Hb=12.9 g/dL, PLT=243,000/µL), peripheral blood smear – normal, transaminases – normal values (AST=15 U/L, ALT=19 U/L), renal function – normal values (BUN=33 mg/dL, creatinine = 0.41 mg/dL) and immunogram test – normal values (IgA=130.1 mg/dL, IgG=1230 mg/dL, IgM=98 mg/dL). Some viral infections were serologically excluded: HBs antigen and HCV antibodies were negative, syphilis serologic test was also negative, Borellia burgdorferi antibody was negative, Quantiferon TB test was negative. The EBV IgG antibodies were positive (11 U/ml) and the EBV IgM antibodies were within the normal values.
The abdominal ultrasound describes two well-defined, 15/26 mm in diameter, lymph nodes in the hepatic hilum, and the presence of some lymph nodes at the base of the mesentery, with a maximum size of 2.5/3.5 mm.
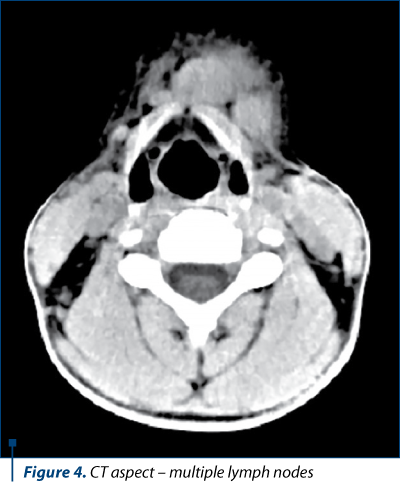
The clinical appearance of lymph nodes required a cervical soft tissue ultrasound and a cranial, neck and thorax CT scan, native and with i.v. contrast, which revealed: non-confluent, rounded, variable sized, deep cervical lymphadenopathy with homogenous contrast substance distribution, the largest lymph node was in the left part and below 1 centimeter; in the right superficial area, one lymph node, similar as morphologic characteristics with the deep ones, but with 1 cm short axis length; non-confluent, rounded, variable in size, supraclavicular lymphadenopathy and axillary non-confluent bilateral lymph nodes, some with lipomatous central degeneration, normal parotids on CT scan and no mediastinal lymph nodes.
Due to anamnestic data that could raise the suspicion of Bartonella henselae infection, the serologic tests (both IgG and IgM antibodies) anti-Bartonella henselae had been performed and the serological titer was positive for IgG antibodies (the obtained value was 1:1024, the reference value being 1:128) and negative for the IgM antibodies. The serologic confirmation of Bartonella infection came to us after 10 days, during which the lymph nodes were reduced in size and number, the abdominal adenopathy disappeared under antibiotic therapy and NDAIS, and the child no longer presented any symptoms.
Discussion
Bartonella henselae is a Gram-negative bacterium, aerobic, pleomorphic, which causes the cat-scratch disease, a common cause of chronic lymphadenopathy among children and adolescents. The responsible vector is the cat, transmitting the pathogen through the bite or scratch. Lymphadenopathy occurs most commonly in the cervical and axillary ganglia, and the infection is self-limiting, usually healing within a few weeks. In healthy children, the prevalence for the ganglia to measure more than one centimeter is estimated between 38% and 45% of all cases. Lymph nodes with a short axis greater than 10 mm are considered abnormal, with the exception of deep cervical lymphadenopathy, in which the diameter of 15 mm is within normal limits.
Almost 75% of patients with cat-scratch disease evolve without complications, less than 50% of them having only fever(2-4). In some cases, however, lymph nodes may confluate, with a tendency to necrosis, fever and severe pain, with the impossibility of swallowing, or more rarely by cardiopulmonary, hepatosplenic and renal involvement(5). In the past, the diagnosis was based on clinical criteria, the history of cat scratching, the skin reaction arising from it, local lymphadenopathy and histopathological outcome. Subsequently, serology became the first-intent test, using the direct immunofluorescence technique for the detection of IgG and IgM antibodies. The laboratory diagnosis of cat-scratch disease (CSD) is based on the determination of specific anti-Bartonella henselae antibodies by different techniques. The Centers for Disease Control and Prevention (CDC) recommends IgG by immunofluorescent assay (IFA) as the gold standard in diagnosing the past infection.
There are studies in literature that have set out to determine the accuracy and utility of anti-B. henselae IgM antibodies by IFA for CSD, but the results have established once again the usefulness of anti-Bartonella henselae IgG(6). Due to its specificity and sensitivity, PCR has been proposed more recently as the B. henselae DNA detection method, but is limited by the need for invasive sampling, such as lymphadenectomy or biopsy(7).
The treatment remains controversial, depending on the status of the disease. Studies have shown that the use of antibiotic therapy significantly reduces the duration of symptoms, the most effective being penicillins, tetracyclines, cephalosporins and aminoglycosides. Ciprofloxacin and azithromycin have been shown to decrease the volume of adenopathy compared to the absence of antibiotic treatment.
Conclusions
Cervical lymphadenopathy requires multiple investigations, using pre-established protocols. Infectious causes, although common in pediatric practice, require a careful clinical examination, detailed medical history, imaging investigations, and often serological confirmation. Until serological results are obtained, which are often late, the treatment is guided by the clinical experience, and the anamnesis can be of real help. When Bartonella henselae infection is suspected, Ig type IgG dosing is the gold standard. Antibiotic treatment for about 10 days is necessary for a rapidly favorable evolution.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
- Dragomir M. Evaluation and management of lymphadenopathy in child: when should a malignity be suspected? Ro J Pediatr. 2018;67(1):13-17.
- Lang S, Kansy B. Cervical lymph node diseases in children, GMS Current Topics in otorhinolaryngology. Head and Neck Surgery. 2014;13:1-27.
- Deosthali A, Donches K, DelVecchio M, Aronoff S. Etiologies of Pediatric Cervical Lymphadenopathy: A Systematic review of 2687 Subjects. Global Pediatric Health. 2019;6:1-7.
- Spijkers S, Littooij AS, Nievelstein RAJ. Measurements of cervical lymph nodes in children on computed tomography. Pediatric Radiology. 2020;50:534-542.
- Ridder-Schröter R, Marx A, Beer M, Tappe D, Kreth HW, Girschick HJ. Abscess-forming lymphadenopathy and osteomyelitis in children with Bartonella henselae infection. Journal of Medical Microbiology. 2008;57:519-524.
- Abarca K, Winter M, Marsac D, Palma C, Contreras AM, Ferrés M. Accuracy and diagnostic utility of IgM in Bartonella henselae infections. Rev Chilena Infectol. 2013 Apr;30(2):125-8.
- Allizond V, Costa C, Sidoti F, Scutera S, Bianco G, Sparti R, Banche G, Dalmasso P, Cuffini AM, Cavallo R, Muzzo T. Serological and molecular detection of Bartonella henselae in specimens from patiens with suspected cat scratch disease in Italy: A comparative study. PLoS One. 2019;14(2):1-11.





