On the 2nd of December 2003, in the Official Journal of the European Union it was published the “Council Recommendation on cancer screening” which had issued recommendations, setting out principles of best practice in the early detection of cancer. The recommendations called on all EU countries to take a common action to implement national, population-based screening programs for breast, cervical and colorectal cancer, as an integral part of the Community Agreement duties in order to complete the national policies, with the aim to improve the public health. A first report, published in 2008, showed that, despite the documented progress, the member states had fallen short of the established target set for the minimum number of examinations by more than 50%. The second report, consisting in the European Council’s recommendations implementation concerning cancer screening, was elaborated and published in 2017, with the attendance of 28 member states. In a subsequent report regarding the European Council’s recommendations implementation status, Romania appears in the minority of EU member states that do not have yet organized population-screening programs for breast cancer and colorectal cancer. In 2020, Romania declared a 0.2% coverage rate of breast examination, an inviting rate of 0.2%, and an 82% participation rate for breast cancer screening. Regarding cervical cancer screening, the coverage rate is 9.2%, the inviting rate is 65% and the participation rate is 14.2%. The general intent of European Union’s plan to combat cancer subsides in the prevention, detection and treatment improvement, as well as cancer management in the EU, reducing health inequalities between and inside the member states. The technical support offered to the member states could contribute to the increase of screening rates, while guides and structural support could contribute to assuring a comparably high health system quality in all Europe.
Actualizare asupra situării României în contextul recomandărilor Uniunii Europene privind screeningul cancerului genito-mamar
Update on Romania’s position in the context of European Union’s recommendations on genital and breast cancer screening
First published: 28 septembrie 2021
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/Gine.33.3.2021.5313
Abstract
Rezumat
La data de 2 decembrie 2003, în Jurnalul Oficial al Uniunii Europene, a fost publicată „Recomandarea Consiliului privind screeningul pentru cancer”, în care Consiliul European dispune dezvoltarea şi implementarea, în ţările membre, ale programelor de screening pentru cancerul cervical, mamar şi de colon, ca parte integrantă a obligaţiilor Tratatului comunitar privind completarea politicilor naţionale cu scopul îmbunătăţirii sănătăţii publice. Un prim raport, publicat în 2008, a arătat că, în ciuda progreselor înregistrate, statele membre nu şi-au îndeplinit obiectivul stabilit pentru numărul minim de examinări, cu mai mult de 50%. Al doilea raport de implementare a recomandărilor Consiliului European privind screeningul pentru cancer a fost elaborat şi publicat în anul 2017, cu participarea a 28 de state membre. Într-un raport ulterior privind stadiul implementării recomandării Consiliului European, România figurează printre puținele state membre ale UE care nu au încă programe populaționale de screening organizat pentru cancerele de sân și colorectal. În anul 2020, România a declarat o rată de acoperire cu examinare mamografică de 0,2%, o rată de invitare de 0,2% şi o rată de participare la screeningul pentru cancer mamar de 82%. În privinţa screeningului pentru cancerul cervical, acoperirea cu examinare a fost de 9,2%, rata de invitare a fost de 65%, iar rata de participare a fost de 14,2%. Obiectivul general al planului UE de combatere a cancerului este reprezentat de îmbunătăţirea prevenirii, depistării, tratamentului şi gestionării cancerului în UE, reducând inegalităţile de sănătate între şi în interiorul statelor membre. Sprijinul tehnic acordat statelor membre ar putea contribui la creşterea ratelor de screening, în timp ce ghidurile şi suportul structural pot contribui la asigurarea unui nivel similar ridicat de calitate în toată Europa.
Cancer remains one of the main death causes in the European Union (EU) and in the entire world. Although cancer is determined by a combination of different factors, including genetic predisposition, environmental influences, lifestyle and infectious agents, avoiding the known risks and adopting a healthy lifestyle could highly decrease the risk of developing a neoplasia. Therefore, prevention is the simplest and the most efficient manner to reduce cancer in the EU.
Cancer screening can be achieved using two strategies:
Population-based screening programs, with screening invitation systematically addressed by the public authorities to one certain segment of the population defined as target, by releasing a political and public document which stipulates the key methods of screening examination.
Opportunist screening, nonpopulation-based, available at request or at medical care providers’ recommendation.
The first “European against Cancer Plan”, dated in the late 1980s, led to new European legislative aspects adoption in relation to tobacco and professional healthcare. Since then, the member states of the EU initiated a series of actions and made a commitment, in accordance with the United Nations Sustainable Development Goals, to reduce the premature mortality due to chronic diseases, including cancer, with a third until 2030. The commitment also included accomplishing the World Health Organization’s (WHO) purposes concerning noninfectious diseases, by reducing with 25% the mortality caused by cancer.
On the 2nd of December 2003, the Official Journal of the European Union has published the “Council Recommendation on cancer screening”(1). According to the EU estimates from 1998, out of 1,580,096 new cases of cancer, 1.4% were caused by cervical cancer, 13% were caused by breast cancer, 14% were located colorectal and 9% interested the prostate, cervical and breast cancer representing 3% and respectively 29% of new cancer involving women. The European Council decided the development and implementation in member countries of screening programs for cervical, breast and colorectal cancer, in accordance with the national legislation, regional and national responsibilities towards the organization and healthcare services provision, as integrative part of the Community Agreement’s Tasks regarding national policies completion with the aim to enhance the public health, to prevent diseases and to recognize the factors that endanger the human health. The screening principles as a prevention method for chronic noncommunicable illnesses have been published in 1968 by WHO and by the European Council in 1994, relying on the “Recommendations on cancer screening” conducted by the Advisory Committee on Cancer Prevention, and on the gained experience during the evolution of the “Europe against Cancer” program, in which there have been established the highest quality standards and good practice guidelines which have the role to administer the screening application. The screening permits cancer detection in a preinvasive stage or in premature invasive stages, so that lesions can be adequately treated, increasing the curability chance. The most important indicator of the screening performance is represented by the decrease of the pathology-specific mortality index; in the case of cervical cancer, identifying precursor lesions induces the reduction of cervical cancer incidence, which makes this parameter an extremely helpful indicator. In order to ensure proper organization and quality control, the member states should allocate adequate human and financial resources, while ensuring equal, equitable access to screening, taking into account the special needs of particular socioeconomic groups included in the target population.
The screening methods that fulfilled the criteria recommended by the European Council in 2003 were: PAP cytological examination for cervical cancer precursor lesions screening, the screening beginning at 20 years of age, but not later than the age of 30; mammographic screening for breast cancer, offered to women aged 50 to 69 years old, in accordance with the European guidelines regarding the quality assurance in mammography; the evaluation of fecal occult blood test as a screening method for colorectal cancer in men and in women aged 50 to 74 years old.
The recommendations of the European Council provide for the use of these screening methods only in the context of organized population, national or regional programs, ensuring quality at all levels, assuring information regarding the benefits and risks, suitable resources for screening, monitoring with complementary diagnostic procedures, psychological support and treatment availability of individuals with positive screening tests. The recording and management of the data obtained through screening is established using high-performance computerized data centralization systems, ensuring the participation of all the implicated subjects through invitations made by the call/recall system. The resulting data must be collected and evaluated until the final diagnosis; data storage and management are recommended to adhere to the standards defined by the European Network of Cancer Registries, and the screening programs control should be performed at an appropriate time interval. The introduction of new screening tests can be done only after their evaluation in randomized controlled trials in order to prove the benefits of the new method on specific screening parameters, on mortality, in the context of consecutive therapeutic procedures, clinical results, side effects, morbidity, quality of life and, last but not least, on the basis of cost-effectiveness(1).
A first report published in 2008 revealed that, despite the progress accomplished, the member states had not reached their objective for the minimum number of examinations, by more than 50%(2).
Between 2009 and 2013, the European Commission implemented the “European Partnership for Action against Cancer” (EPAAC), funded as a joint action under the EU Health Program, with the aim of promoting and supporting the development of the National Cancer Control Plans in the member states. In the policy documents developed in this joint action, a special attention was paid to cancer screening programs, highlighting the significant impact that these screening programs had on health indicators, especially on reducing the cancer incidence and mortality. On this occasion, the European Commission adopted the Communication to the European Parliament, the Council of Europe, the European Economic and Social Committee and the Committee of the Regions on the European Action against Cancer: the European Partnership. This report presents the European progress towards the stated goal of the European initiative to reduce the incidence of cancer with 15% by 2020. The EPAAC joint action was followed by the CANcer CONtrol joint action (CANCON), which was finalized in February 2017 with the publication of a European Guide to Cancer Plans and a set of health policies which continue to support the organization of population-based cancer programs for the three tumor sites, which must be implemented in accordance with the European Guidelines for Quality Assurance in the screening programs, which define the organization and implementation planning, so that these programs provide maximum benefits and minimum risks.
The second report on the implementation of the European Council Recommendations on cancer screening was elaborated and published in 2017, with the participation of 28 member states(3). The implementation analysis of the 2003 European Council screening recommendation in the second report revealed that breast cancer screening program was materialized in 25 of the 28 member states, with 95% evaluated women aged 50-69 years old, and cervical cancer screening was ensured in 22 of the member states, covering approximately 72% of the target age group 30-59 years old, with huge discrepancies between the member states in terms of implementation status and the screening expansion, with the report’s purpose to improve performance by reviewing each level of implementation.
In 2012, according to the International Agency for Research on Cancer (IARC) estimates, in Europe cancer was responsible for 1.26 million deaths in the 28 states that participated in the implementation of screening, with breast cancer alone causing 91,500 cases of mortality, and cervical cancer causing another 13,000 deaths. In this second cause of population mortality after cardiovascular diseases, 40% of deaths are considered to be preventable; however, across Europe, only an average of 3% of health budgets are allocated to prevention, although the role of prevention measures and screening is well known. The cancer screening programs implementation in EU member states represents the most comprehensive prevention action in order to reduce breast, cervical and colorectal cancer mortality, as well as reducing the incidence of cervical and colorectal diseases. The report identifies the implementation extend and also an essential series of indicators that must be continuously monitored to ensure improved screening quality, which are especially useful to gradually expand the program coverage and to provide a basis for enhancing screening effectiveness in the EU. Data quality can be improved by introducing potent health information systems capable to associate screening programs to the existing cancer and mortality registries(3).
The European Committee has funded and supported the development of European Guidelines for Quality Assurance in scientific evidence-based cervical, breast and colorectal cancer screening. These guidelines are constantly reviewed and updated, incorporating all existing scientific documentation. Thus, the European Guidelines for Quality Assurance in Cervical Cancer Screening has reached its second edition (2015), and the European Guidelines for Quality Assurance in Breast Cancer Screening was revised in 2013(4-7).
The European Commission has also created the European Breast Cancer Initiative and the European Colorectal Cancer Initiative, groups of experts whose role is to provide recommendations and guidance on quality assurance services for the detection, diagnosis and treatment of breast and colorectal cancer.
Romania has been completely deficient in nationally organized cancer screening programs until 2012, when, after a regionally conducted pilot program in the Northwest region, the national program for the early active detection of cervical cancer was initiated, using Babeş-Papanicolaou testing in screening regime financed from the Ministry of Health budget. Since 2016, Romania has embraced a project called National Cancer Control Plan (PNCC). This guide represents one of the testing strategies and includes screening programs recommended by the Oncology Commission of the Health Ministry; it has been developed in 2009-2016, as a specific chapter on secondary prevention from the National Cancer Control Plan. The strategies contained in the PNCC were developed within the European Cancer Action Partnership (EPAAC) Joint Action and were finalized and publicly communicated in 2016 under the CANCON Joint Action.
In the latest report concerning the implementation situation of the European Council Recommendation (2003/878 /EC), Romania is among the few EU member states that do not yet have organized population screening programs for breast and colorectal cancer(3). The coordination of an organized breast cancer screening program was piloted in the North-West region, within a project funded by the financial mechanism Norway-EEA, but the results were not recovered and developed into an extending program. Thus, according to the data centralized by “Cancer Screening in the European Union Report on the Implementation of the Council Recommendation on cancer screening” from 2017, Romania had no reported data regarding the population screening for breast cancer, respectively the diagnosis, treatment and evolution of cases(3). Regarding breast cancer, for the analyzed period (2012-2013), in the target population, consisting in approximately 1.3 million individuals, the invitation rate and the examination rate were 0.2%(3). This 0.2% represents invited target population to participate to the screening program, respectively 3000 women, with a participation and examination rate of 82%, respectively 2460 tests corresponding to Cluj County(3); no further results communications exist for the 2460 people reported as included in the screening program(3).
Regarding cervical cancer screening, the reporting of addressed and examined subjects is between 0% and 11%(3), taking into account that in 2018 the cervical cancer cases accounted for approximately 9% of all cases of cervical cancer. According to the reported data, the screening program included a population from all the age groups, with a target number of 1,127,544 women to be invited, of which 733,010 were invited to the screening program, representing 65%, without reporting the distribution by age groups(3). Of the same number of women representing the target population, 103,886 women were effectively included in the screening and examined, respectively 9.2%(3). By summarizing the presented data, the screening participation rate was 14.2%(3).
The European Union’s latest report dates from July 2020(8). In numbers, the low coverage and wide variability of cancer screening implementation among European countries transposes into a mammographic examination coverage rate between 17% and 84%, a variable cervical cancer examination coverage rate between 4% and 71%, and a colorectal cancer screening rate of 1-53%. Romania declares a mammographic examination coverage rate of 0.2%, the invitation rate being 0.2% and the breast cancer screening participation rate being 82%. Regarding cervical cancer screening, the examination coverage has a 9.2% rate, the invitation rate was 65% and the participation rate was 14.2%, the latter report being in contradiction with the participation rate reported for breast cancer screening, which raises the issue of validity of reporting participation rate for breast cancer screening(8).
The National Health Strategy 2014-2020, assumed by Government Decision no. 1028/2014, mentions among the major public health problems the fact that, in the female population, breast and cervical cancer constitute the malignancies with the greatest impact on premature mortality (before 65 years old), with over 70,000 and 50,000 potentially lost years of life through premature deaths each year. The document emphasizes that “Romania performs suboptimal in the prevention field, including early detection of cervical cancer, the mortality from this disease is increasing or at most it remains stable” and that “the recently established national screening program for cervical cancer requires a few good years of implementation, sustained funding, increased performance according to specific standards before the appearance of the first significant signs of stable impact on mortality”. It is also emphasized the fact that the high morbidity and mortality rates make cervical cancer primary prevention by vaccination against human papillomavirus (HPV) a highly relevant and fundamental intervention in Romania, especially as strains 16 and 18 of HPV are responsible for about 70% of cervical cancer cases.
Primary and secondary prevention efforts for preventable cancers are addressed in two specific objectives on cervical cancer in the national health strategy:
Strengthening/developing the capacity to manage and/or implement the vaccination program according to the current national calendar and ensuring the necessary resources for an improved national vaccination schedule (including the extension of HPV vaccination) (Objective 2.2.), an intervention involving cervical cancer primary prevention.
Reducing the cancer burden in the population by detecting the early stages of the disease and reducing the medium-term or long-term specific mortality through organized screening interventions (Objective 3.2), an intervention involving secondary prevention by early active diagnosis of preventable cancers.
National health programs include certain approaches to the cervical cancer primary and secondary prevention. The cervical cancer screening subprogram is monitored by technical assistance units (TAUM), with territorial division, according to Table 1. The budget allocated to the National Program is presented in Table 2.
In the period 2014-2020, the Human Capital Operational Program (HCOP) accomplished two screening projects: “Integration of primary HPV screening in the national screening program for cervical cancer” (SMIS Code: 120798) and “Increasing the institutional capacity and professional competencies of health system specialists in order to implement the National Breast Cancer Screening Program” (SMIS Code: 120799).
Each of these two projects has a phase 2, which is in the evaluation stage, as follows:
a) For cervical cancer screening – it is intended to select and fund four projects, covering the regions North-West, Center, South Muntenia and North-East. The program offers Babeş-Papanicolaou testing for women aged 25-29 years old and HPV testing for women aged 30-64 and, if positive, cytological triage for women aged 30-64, respectively HPV testing for women aged 25-29 and for uninsured women, early diagnosis and treatment of dysplastic lesions. Each project should include at least 170,000 eligible women, which would be equivalent to 20% of the female population eligible for screening.
b) For breast cancer screening – it is intended the selection and funding of two projects, covering the regions North-West, West (the first project), respectively North-East and South-East (the second project). The program offers double reading and arbitration mammography testing for women aged 50-69 years old; each project should include at least 15,000 eligible women, which would be equivalent to about 2% of the female population eligible for screening.
The presence of strategic and operational interventions for the three pathologies is summarized in Table 3.
We can affirm that cervical cancer is found in national policies and programs, but of the other two pathologies of interest, breast cancer is only mentioned as a public health issue in the strategy, and at the moment there is only a stated intention – “The Ministry of Health is designing a gradual inauguration of pilot screening projects for breast cancer in women and colorectal cancer in both sexes”. The early detection of breast cancer is not included among the screening programs, there is only a national program supported by the National Health Insurance System, including breast reconstruction after surgery.
The Activity Report of the Ministry of Health for 2018 (last available) declares that, during this year, 116,253 HPV vaccinations were performed on at-risk population group (girls aged 11-14 years old). Thus, if we consider that an annual cohort of girls has a number of about 100,000 people and that each girl should have three vaccine doses, we can estimate that in 2018 the HPV vaccine coverage was below 10%.
Regarding the screening of cervical cancer, the same report of the Ministry of Health states that the screening subprogram in 2018 operated with 75 networks, which have performed tests according to Table 4.
The incidence of the two types of pathologies in 2018 is presented in Table 5.
The standardized mortality for the two pathologies in Romania compared to the European Union is represented in Figures 1 and 2. Based on the analysis, we conclude that the progress made by Romania since the beginning of the implementation of the national health strategy 2014-2020 in terms of health status on breast and cervical cancer is modest, meaning that the annual incidence remains above the European rate (Europe WHO), exceeding majorly on cervical cancer (+54%), with Europe conjoining all 53 member states, including Eastern European states, where healthcare is traditionally poorer.
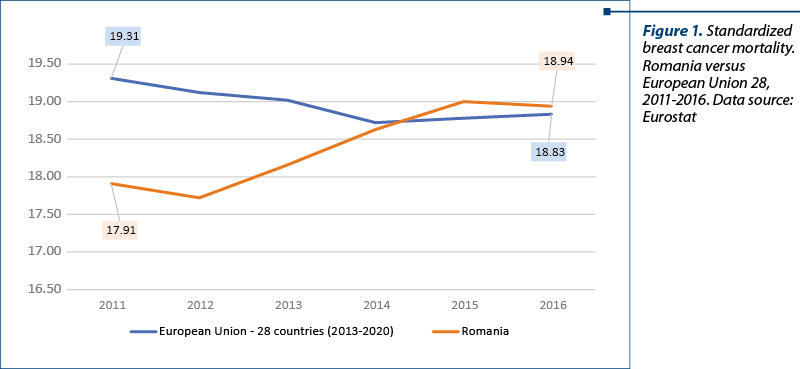
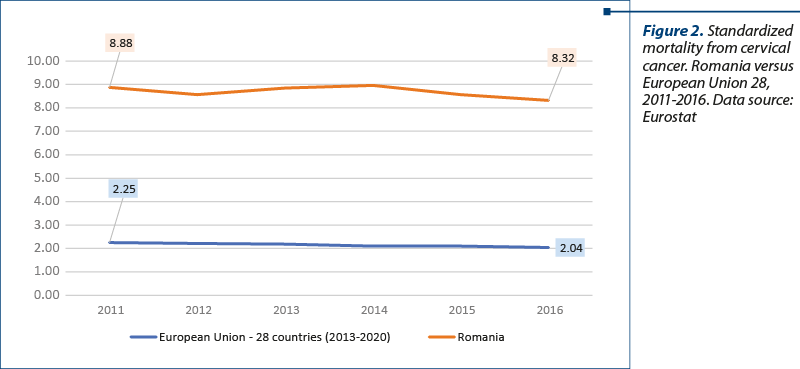
The incidence analysis in Globocan is based on estimates, given the fact that in Romania it is reported only through the regional registers Cluj and Timişoara. For this reason, we chose the EU-Eurostat database for the mortality analysis. Especially in the case of mortality, there is an increase in mortality caused by breast cancer, while in the EU there has been reported a decrease and respectively stagnation for the other two pathologies. The standardized rate of cervical cancer mortality is four times higher in Romania than the EU average, cervical cancer being a preventable cause of death.
Starting with the 2003 recommendations, the scientific and technological progress has added new increased performance methods for breast, cervical and colorectal cancer screening; digital mammography, digital breast tomography, or nuclear magnetic resonance as a complementary method for women with extremely dense breast tissue, HPV testing, fecal immunological testing or endoscopy are progressively implemented within the European Union program. The results obtained in the field of neoplastic risk prediction announce a new development strategy: screening based on risk stratification.
The new European Beating Cancer Plan focuses on all key disease strategies: prevention, early diagnosis, treatment, nursing and increasing patients’ quality of life. Based on the support of the member states, the European Parliament leaders, including members of the MAPs Against Cancer group who have worked alongside the European Committee to enhance cancer prevention and nursing in Europe, will complete the existing national cancer plans in each European country. The development of the EU Cancer Plan will take place in close proximity to Mission on Cancer, a new initiative of the Horizon Europe Framework Program for Research and Innovation, which maximizes the impact of European support for research and innovation and demonstrates the relevance of their results towards society.
Issues caused by cancer have a wide range of affects. In addition to causing enormous pain and physical and emotional suffering, cancer is also a burden on health systems and society generally, with the economic impact of cancer in the EU estimated at over €100 trillion a year.
Healthcare systems were originally designed to treat short-term, acute infectious diseases. Given the general aging of the population, the growing prevalence of noncommunicable and emerging diseases and patterns changing, cancer continues to be the main candidate with increasing demands for healthcare and associated costs. The global economic impact of cancer in Europe is estimated at more than €100 billion a year. Increased pressure on national health and social care systems is detrimental to the focus on the patient and on continuing healthcare, which in case of cancer requires increased interdisciplinary collaboration in the health sector and cooperation with other sectors. Currently, the concept application of the treatment team composed of radiologist, surgeon, oncologist, healthcare staff, physiotherapists and researchers has limited applicability, occurring only in excellence centers. It is therefore clear that there is an urgent need for an outstanding focus on disease prevention and that the relevant skills of healthcare professionals should be strengthened. In some countries, a holistic approach is also essential in forming health workers, which also involves non-medical staff and informal caretakers, to elevate, for example, palliative care and pain management. The development of a coordinated patient-centered approach must overcome the gaps between health and other sectors, connecting the hospital with primary care and community social services, helping patients to navigate the system and receive ongoing care. In addition, in many member states there are general deficiencies and discrepancies in training the healthcare workforce (including cancer specialists), with the future plan requiring solutions to these problems.
The general mission of the EU Cancer Plan is to improve cancer prevention, detection, treatment and management in the EU by reducing health inequalities between and within the member states. It will establish actions that support, coordinate or complete the efforts of the member states. In terms of early diagnosis, it is essential to reduce the time necessary until diagnosis, increase the coverage of the target population for breast, cervical and colorectal cancer screening, thus providing evidence-based indications to expand cancer screening to other cancers (lung, prostate, stomach), prostatic specific antigen and low-dose computed tomography being currently under investigation.
The technical support offered to the member states could aid in increasing screening rates, while guidance and structural support can sustain a similarly high-quality level across Europe. Cancer Mission, European Health Data Space and European Cancer Knowledge represent proposals for new program implementations focused on the applicability of digitization and artificial intelligence which will be helpful in processing enormous amounts of data that will increase the apprehending of disease mechanisms and the development of new therapies.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
- European Council. Council recommendation of 2 December 2003 on cancer screening (2003/878/EC). Off J Eur Union. 2003;327:34–8.
- von Karsa L, Anttila A, Ronco G, Ponti A, Malila N, Arbyn M, Segnan N, Castillo-Beltran M, Boniol M, Ferlay J, Hery C, Sauvaget C, Voti L, Autier P. Cancer screening in the European Union. Report on the implementation of the Council Recommendation on cancer screening – First Report. European Commission, Office for Official Publications of the European Communities, Luxembourg, 2013. Available at: http://ec.europa.eu/health/ph_determinants/genetics/documents/cancer_screening.pdf
- Ponti A, Anttila A, Ronco G, Senore C. Cancer Screening in the European Union. Report on the implementation of Council Recommendation on Cancer Screening. Brussels: European Commission, 2017. Available at: https://ec.europa.eu/health/sites/default/files/major_chronic_diseases/docs/2017_cancerscreening_2ndreportimplementation_en.pdf (Accesed on 21 March, 2021).
- Perry N, Broeders M, de Wolf C, Tornberg S, Holland R, von Karsa L. European guidelines for quality assurance in breast cancer screening and diagnosis. 4th ed Suppl. European Commission, Office for Official Publications of the European Union, Luxembourg, 2013. Available at: https://op.europa.eu/en/publication-detail/-/publication/4e74ee9b-df80-4c91-a5fb-85efb0fdda2b
- Arbyn M, Anttila A, Jordan J, Schenck U, Ronco G, Segnan N, Wiener H, Herbert A, Daniel J, von Karsa L (eds.) European guidelines for quality assurance in cervical cancer screening. 2nd ed. European Commission, Office for Official Publications of the European Communities, Luxembourg, 2008. Available at: http://bookshop.europa.eu/is-bin/INTERSHOP.enfinity/WFS/EU-Bookshop-Site/en_GB/- /EUR/ViewPublication-Start?PublicationKey=ND7007117.
- Perry N, Broeders M, de Wolf C, Törnberg S, Holland R, von Karsa L, Puthaar E (eds.) European guidelines for quality assurance in breast cancer screening and diagnosis. Fourth edition. European Commission, Office for Official Publications of the European Communities, Luxembourg, 2006. Available at: http://bookshop.europa.eu/is-bin/INTERSHOP.enfinity/WFS/EU-Bookshop-Site/en_GB/- /EUR/ViewPublication-Start?PublicationKey=ND7306954
- von Karsa L, Arbyn A, De Vuyst H, Dillner J, Dillner L, Franceschi S, Patnick J, Ronco G, Segnan N, Suonio E, Törnberg S, Anttila A. Executive summary. In: European guidelines for quality assurance in cervical cancer screening. 2nd Ed, Suppl. Anttila A, Arbyn A, De Vuyst H, Dillner J, Dillner L, Franceschi S, Patnick J, Ronco G, Segnan N, Suonio E, Törnberg S & von Karsa L (eds.). Office for Official Publications of the European Union, Luxembourg, 2015.
- Couespel N, et al. Strengthening Europe in the fight against cancer, study for the committee on Environment, Public Health and Food Safety, Policy Department for Economic, Scientific and Quality of Life Policies, European Parliament, Luxembourg, 2020. Available at: https://www.europarl.europa.eu/RegData/etudes/STUD/2020/642388/IPOL_STU(2020)642388_EN.pdf
Articole din ediţiile anterioare
Cancerul colorectal în sarcină
Incidenţa cancerului colorectal în timpul sarcinii este mică, de un caz la 1. 000 de sarcini. Cancerul mamar, ovarian şi cel cervical sunt cele mai...
Leziunile CIN de grad înalt în sarcină şi post-partum: o asociere rară. Noutăţi în abordare
Scopul acestei lucrări este de a reevalua oportunitatea terapiei leziunilor CIN 2/3 diagnosticate în cursul sarcinii, pentru a evita progresia a...
Markeri moleculari utilizaţi pentru diagnostic, prognostic şi terapie în cancerul mamar - review
Lucrarea îşi propune actualizarea informaţiilor privind implementarea în practica clinică a markerilor moleculari necesari stabilirii diagnosticulu...