Clinical and surgical staging in patients with cervical cancer – a retrospective study regarding correlations between initial diagnosis, treatment options and histopathological results
Stadializarea clinică şi chirurgicală a pacientelor cu cancer de col uterin – studiu retrospectiv privind corelaţiile dintre diagnosticul iniţial, opţiunile de tratament şi rezultatele histopatologice
Abstract
According to the International Federation of Gynecology and Obstetrics (FIGO), cervical cancer staging is realized through clinical examination. The imaging and subsequent surgical findings come in addition to the first staging. Although in the incipient stages surgery is the first step in treatment, in advanced stages the treatment options are based on the clinical staging in corroboration with imaging techniques. The recognition of the prognostic factors, such as lymph node metastasis and distant metastasis, represents a key element for the proper diagnosis and treatment. In this paper, we present a retrospective study that included patients with cervical cancer for which imaging evaluation of the lymph nodes was performed and was compared with post-surgery histopathological results.Keywords
cervical cancerstaginglymph nodesimagingRezumat
Conform Federaţiei Internaţionale de Ginecologie şi Obstetrică (FIGO), stadializarea cancerului de col uterin se realizează prin examinare clinică. Imagistica şi constatările chirurgicale ulterioare au rolul de a îmbunătăţi precizia diagnosticului faţă de prima stadializare. Deşi în stadiile incipiente intervenţia chirurgicală este primul pas în tratament, în stadii avansate opţiunile de tratament se bazează pe stadializarea clinică, în coroborare cu tehnicile imagistice. Recunoaşterea factorilor de prognostic, precum metastazele ganglionilor limfatici şi metastazele la distanţă, reprezintă un element-cheie în diagnosticul şi tratamentul adecvat. Această lucrare prezintă un studiu retrospectiv ce a inclus paciente cu cancer de col uterin pentru care s-a realizat evaluarea imagistică a ganglionilor limfatici, iar rezultatele au fost comparate cu rezultatele histopatologice postoperatorii.Cuvinte Cheie
cancer de col uterinstadializareganglioni limfaticiimagisticăIntroduction
Cervical cancer represents a common public healthcare issue. Worldwide, it is on the fourth place in the list of common cancers in patients regarding age, and 85% of them are in regions with restricted resources(1). In 2018, the International Federation of Gynecology and Obstetrics (FIGO) updated the 2009 recommendations. The new guideline takes into consideration the day-to-day clinical practice and the needs for treatment stratification. The stage of the cervical cancer determines the treatment options. Surgical treatment and chemoradiotherapy represent the management alternatives.
According to FIGO, the staging for cervical cancer is currently made clinical. There is a strong recommendation that imaging techniques should be used to offer a more accurate staging(2). It is considered that the staging for cervical carcinoma can be imprecise in a significant number of patients with both early and advanced stages(3). The 2018 staging aimed at reducing the rate of error by increasing the importance of imaging. Two main changes were represented by redefining stage IA (removing lateral extension) and stage IB (introducing the 2-4 cm group). For stage III, the presence of lymph node involvement can be indicated by imaging or pathology (notations ‘r’ and ‘p’ should be mentioned)(4).
The correct assessment of lymph nodules is important for proper staging and treatment, as lymph node metastasis, parametrial involvement, stromal infiltration and metastasis in other organs are negative prognostic factors(5-7). Failure to evaluate the extent of the disease can lead to suboptimal treatment and to disease recurrence(8). The presence of paraaortic lymph node metastases is corelated with poor prognosis and with risk for disease recurrence(8,9), and it is encountered in up to 16% of the cases(10). As a solution, the surgical evaluation of nodules in advanced cancer was assessed. Laparoscopic paraaortic lymphadenectomy for advanced cases could help adjust radiotherapy(11). The involvement of other lymph nodes can be present in up to 44% of cases and require adequate chemoradiotherapy in order to obtain a proper response(10).
Although the most precise evaluation is realized by surgical staging, the procedure can be corelated with increased morbidity and mortality when radiotherapy is needed(8). Laparoscopy and robotic surgery can be options to reduce complications(12,13). Surgical evaluation is associated with considerable rates of upstaging(14). There is a potential benefit from surgery that is represented by the metastatic lymph node debulking prior to the other treatments and thus obtaining higher control(6).
As an alternative, imaging assessment of the lymph nodules is encouraged to be made. Computed tomography, magnetic resonance imaging and positron emission tomography can be used in all stages of cancers. When compared with surgical staging, these techniques have lower sensitivity and specificity. There is a false-negative rate regarding the imaging method used for evaluation(9,11,15,16), but it seems that it is no difference between surgical and clinical staging regarding recurrence rate in women with advanced cervical cancer(6).
For every patient, it is important to determine the most favorable management, including diagnosis and treatment. The proper evaluation using adequate imaging can contribute to a more precise staging and adapted medical and surgical treatment. The aim of this paper is to highlight the correlation between imaging aspects and surgical aspects in patients with various stages of cervical cancer.
Materials and method
We performed a retrospective analysis of the patients with cervical malignant tumors investigated in the University Emergency Hospital Bucharest in the last five years (between January 2017 and December 2022). We analyzed the database of the Obstetrics-Gynecology Clinic to obtain information regarding clinical staging of patient, post-surgery staging, and the relevance of imaging in these cases. The aim of this study was to compare the clinical staging and imaging findings with the histopathological forms and lymph node implications. This analysis was part of the national, single-center, investigational, retrospective clinical research study entitled “Surgical treatment methods in cervical cancer” (study number 74825/07.12.2021), carried out in the Obstetrics-Gynecology Clinic of the Emergency University Hospital Bucharest, for a period of five years. The purpose of this project was to study different types of surgical interventions, including lymphadenectomy and new diagnostic procedures, monitoring human papillomavirus (HPV) infection and evaluating lymphedema of the lower limbs after lymph node dissection.
Results
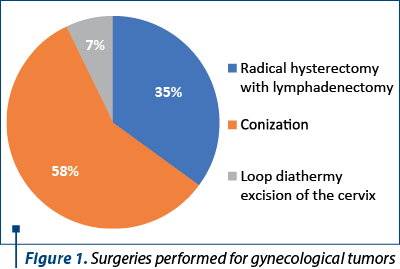
During the studied period, 826 patients were reported with gynecological cancers, and 746 interventions were performed for malignant tumors. There were performed 50 loop diathermy excision of the cervix, 437 conizations and 259 radical hysterectomies with lymph node dissection (Figure 1). In the last category, there were included operations performed for uterine cancer and ovarian cancer as well.
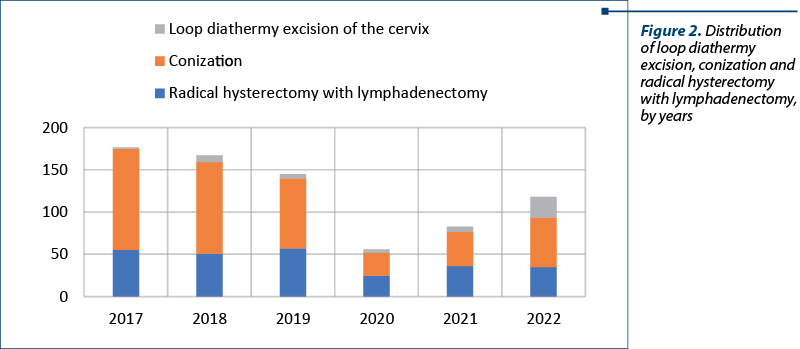
The distribution by year is showing the impact of the COVID-19 pandemic. The lowest number of surgeries were performed in 2020 – a total of 56 procedures (four loop diathermy excision, 27 conizations and 25 radical hysterectomies). As presented in Figure 2, in 2017 there were performed the most procedures (117). The total number started to increase after lifting restrictions, as seen in 2021 (83 procedures) and 2022 (118 procedures).
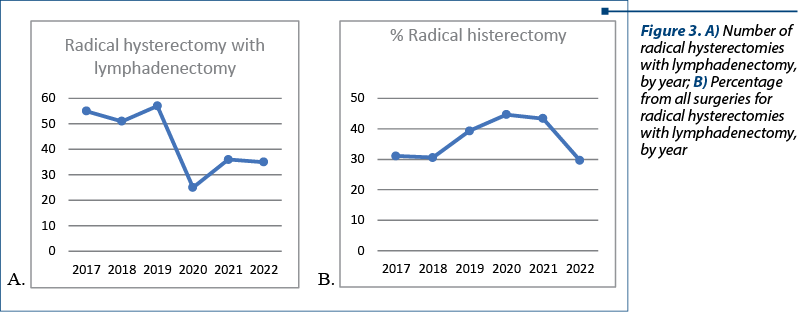
Analyzing the data, we observed that the number of radical hysterectomies with lymphadenectomy dropped during the pandemic but, at the same time, the percentage from the total number of interventions was significantly increased (25 radical hysterectomy with lymphadenectomy, representing 44.6% from all surgeries, compared with a median of 55 radical hysterectomy with lymphadenectomy a year in the previous three years, representing 33%) – Figure 3. These data can be corelated with the diagnosis in advanced forms of cancer rather than the diagnosis of preneoplasia lesions due to delayed presentation. The patients who underwent radical hysterectomy with lymphadenectomy were mostly from urban areas (161 women versus 71 women from rural areas). In this lot of patients, there was one person older than 80 years of age, with 42.67% of patients between 40 and 59 years old, and 51.72% between 60 and 79 years old.
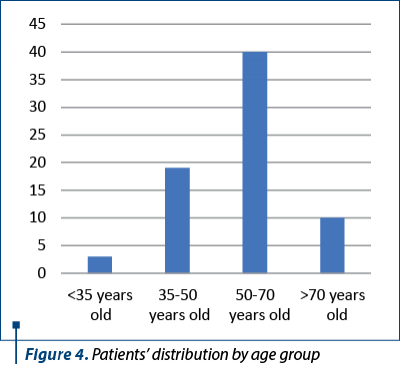
Out of these women who underwent surgical procedures in our clinic, we selected 72 patients who had radical hysterectomy with lymphadenectomy for cervical cancer of different stages. The median age was 58.5 years old and the medium age was 55.8 years old, with the youngest women of 26 years old and the oldest patient of 75 years old. The distribution by age groups is presented in Figure 4. The majority of women came from urban areas (56 patients).
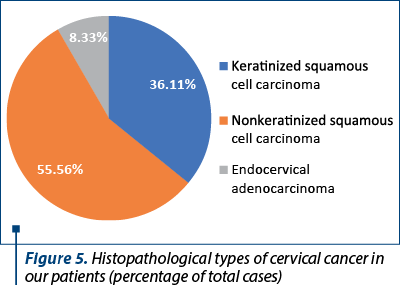
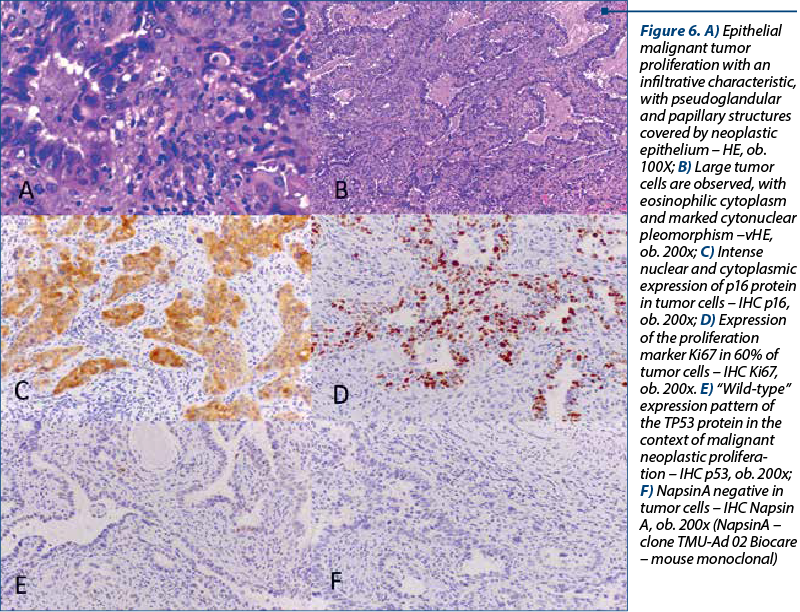
The histological types of cancer encountered were represented by keratinized squamous cell carcinoma (26 patients), nonkeratinized squamous cell carcinoma (40 patients) and endocervical adenocarcinoma (six patients) – Figure 5. One particular case was represented by a 50-year-old patient diagnosed with endocervical adenocarcinoma G3 associated with stratified mucin-producing intraepithelial lesion (SMILE). WHO classifies SMILE as a rare high-grade cervical precancerous lesion and a variant of adenocarcinoma in situ. Figure 6 reveals the histopathological aspects of this particular tumor.
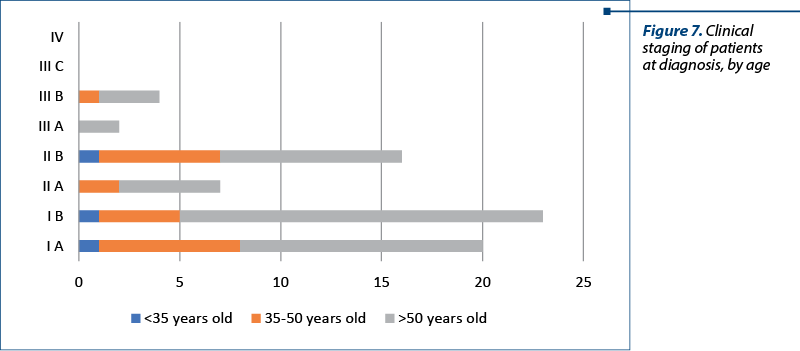
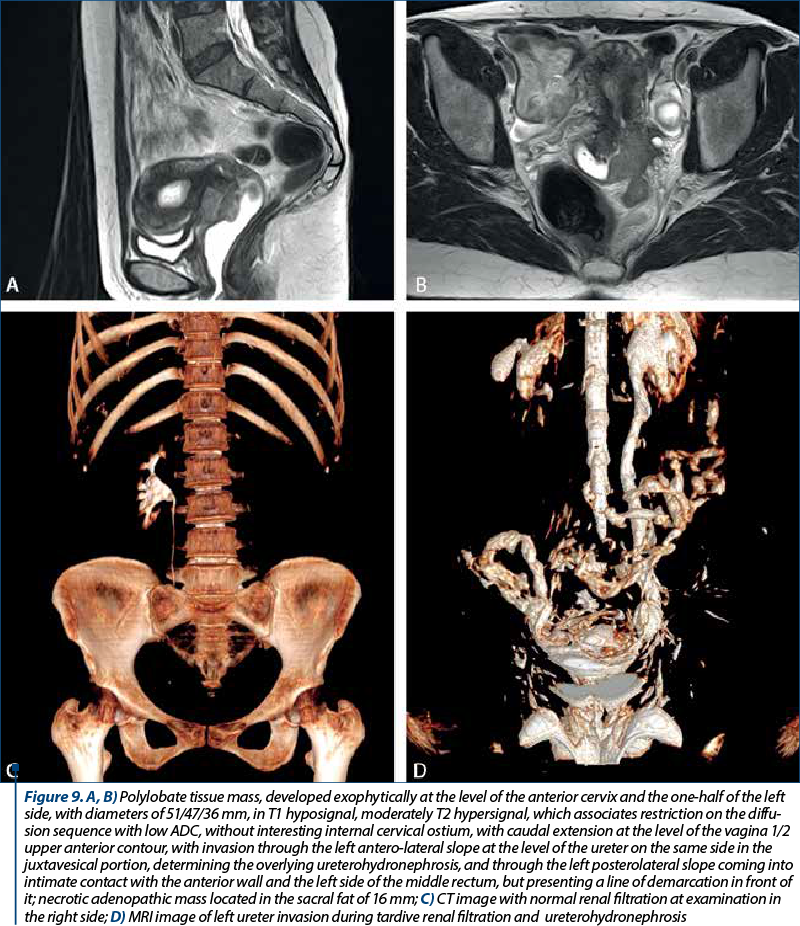
The clinical staging was assessed with the clinical evaluation of the patient and with imaging techniques (CT and MRI). In our studied group, there were 20 patients with stage IA, 23 patients with stage IB, seven patients with stage IIA, 16 patients with stage IIB, two patients with stage IIIA and four patients with stage IIIB. There were no patients with stage IIIC or IV. The distribution of cervical cancer staging by age can be seen in Figure 6. In this analysis, patients with in situ cervical carcinoma or with stage IV were not included.
In order to evaluate properly the patient, the MRI and CT protocols were followed. The patients were required to present with bladder and rectum emptied with 30-60 minutes before the procedure. The purpose was to obtain a partial distention of the bladder and a favorable uterine position. The typical MRI image of cervical tumors reveals intermediate T2-weighted signal intensity and enhanced T1-weighted signal for small tumors.
Out of a total of 72 patients, 44 women (61.11%) underwent chemoradiotherapy, of which 29 patients had no residual tumor at surgery. For 28 patients (38.89%), radical hysterectomy with lymphadenectomy was the first line of treatment. There were four patients who were subdiagnosed and the clinical staging was lower than the surgical staging, although the imaging did now show lymph node implication, parametrial invasion or extension to vagina or uterus. There were six cases with stages IA, IB or IIB in which the recommendation for chemoradiotherapy and not surgery was made, taking into consideration the aspect of the lymph nodes at CT or MRI. In five cases, positive lymph nodes were identified at post-chemoradiotherapy surgery.
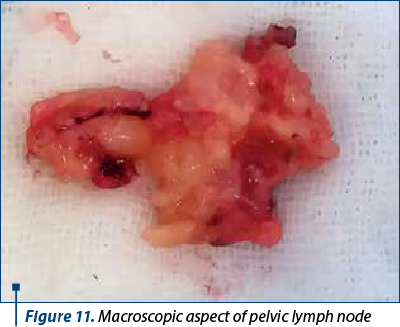
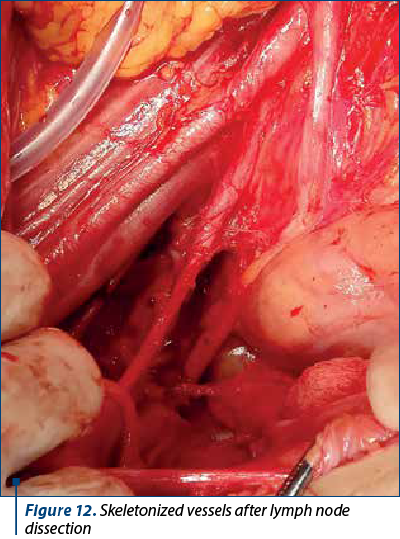
To better evaluate the patient, in all patients it was performed lymphadenectomy that included iliac, crural and obturator nodes. Lymph node dissection is realized in the first surgical steps. The first nodes evaluated are the ones located on the opposite site of the surgeon, near the iliac vessels, followed by the dissection of the retrocrural lymph nodes. After removing this lymph nodes groups, the obturator lymph nodes are removed. The next step is represented by evaluating and removing this groups of nodes from the side of the main surgeon. Figures 11 and 12 show the macroscopic aspects of the pelvic lymph nodes and the skeletonized vessels after lymph node dissection.
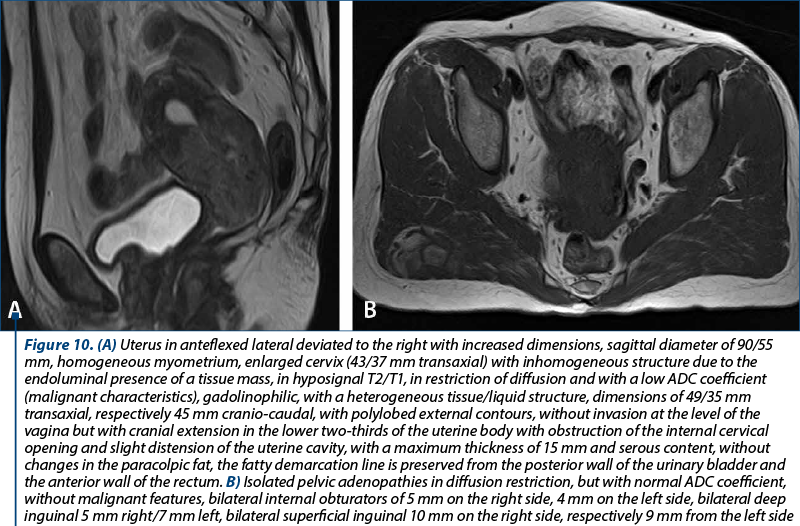
The median number of dissected lymph nodes was 17.5, with a total number of 1206 sampled lymph nodes and 31 nodes with present metastasis. In 22 women, the lymph node aspect was of reactive histiocytosis. For the 28 patients who had primary surgery, a total of 446 nodes were evaluated and the median number of lymph nodes was 14.5. The increased number of lymph node dissection was not associated with higher rates of metastatic lymph node detection. In our group, we encountered two patients who presented lymph node invasion at the histopathological evaluation. Twenty-six patients (92.86%) had no metastatic nodes.
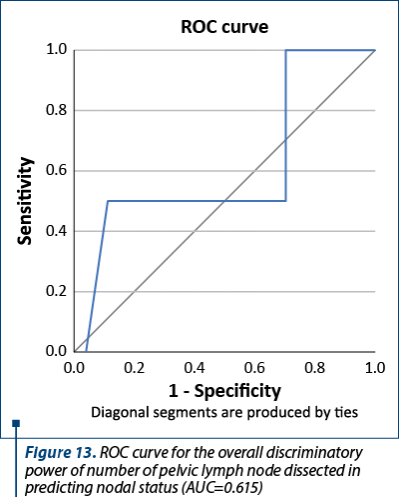
Figure 13 shows the ROC curve for the overall discriminatory power of pelvic lymph node dissection in predicting nodal status with corresponding AUC of 0.615. This value was observed when the lymph node examination was categorized using 15 lymph nodes as cut-off points. The number of lymph nodes was not associated with the pathologic finding of metastatic lymph nodes.
Discussion
Cervical cancer is one of the most prevalent forms of cancer in developing countries and has one of the highest mortality rates worldwide(17). Unfortunately, Romania occupies the first place in Europe regarding mortality in cervical cancer(18). Its prevalence is corelated with low socioeconomic status, low education level and with poor addressability to healthcare. It is considered that the difference between the high-income and low-income regions prevalence is due to the difference in access to screening(17).
After the 2018 FIGO review of cervical cancer staging, a better classification that reflects the clinical practice entered the daily use. The changes helped to estimate in which survival category the patients fit and which is the optimal treatment pathway. Multiple validation studies have shown a statistically significant improvement of five-year survival and disease-free period when the 2018 staging is applied(19-21). In our analysis, the patients included were staged both with 2009 FIGO staging (patients from 2017 and 2018) and the 2018 FIGO staging (patients from 2019-2022). The management of the patients from the first two years of the study with suspicion of lymph node invasion or extension of the neoplasia was similar to that of patients who were staged according to 2018 FIGO staging. In further studies, we aim to re-stage the patients and report the follow-up according to the new stage.
Squamous cell carcinoma of the cervix is the most prevalent tumor and sums up to 80% of the total cervical malignancies(22). A lower percentage of the patients are diagnosed with adenocarcinoma, clear cell carcinoma or serous carcinoma. Although the majority of histopathological types are associated with the HPV infection, adenocarcinomas have been evaluated as non-HPV related cervical cancers(23). In our studied group, 79.17% of women had squamous cell carcinoma and 8.3% of women had adenocarcinoma. In one case, the histopathological diagnosis was endocervical adenocarcinoma with HPV-associated and SMILE (stratified mucin-producing intraepithelial lesion) of the uterine cervix. SMILE is classified as a rare high-grade precancerous lesion(24). The histopathological aspects include cellular stratification the same as in squamous intraepithelial lesions. In the literature, SMILE is associated with invasive squamous carcinoma or adenocarcinoma(25,26) and has been correlated with high-risk genotypes(26,27). In our case, the patient had high-grade endocervical adenocarcinoma, and HPV 16 and 62 were present.
One important aspect in staging and management is represented by the identification of the lymph node involvement. The classic criteria of suspicion of lymph node involvement included the dimension (larger than 10 mm – long axis, or 8 mm – short axis) and shape (round)(28). The imaging techniques can present capsular breach, lymph node necrosis or signal intensity similar to the primary tumor. This are signs of lymph node involvement with high predictive value both in MRI and CT(29). The 2018 FIGO staging increased the relevance of MRI as assessing the main tumor characteristics, parametrial and the lymph node involvement. In the meantime, CT is more frequently used in lower income countries. Although it can be used with high accuracy for advanced stages, it is not accurate without evident parametrial invasion or cervical stroma integrity(30). In our study, the clinical staging was made by correlating the clinical findings with the imaging characteristics of the tumor. In our clinic, the implementation of routine MRI for cervical cancer patients was realized simultaneously with using the new FIGO staging.
The dissection of the lymph nodes is realized by removing the baring fat tissue that is adjacent to the vessels. In the literature, the median number of lymph node dissected varies from 9 to 48 nodes(31-33), decreasing in time(34). In low stages (IA or IB), lymph node involvement is encountered in 3.7% up to 21.7% of the patients(31). In our study, in the group with primary surgery with stages IA or IB, the percentage of lymph node implication was 7.14% out of 28 women. The multidisciplinary evaluation committee recommended chemoradiotherapy followed by surgery for the women with imaging suspicion of ganglion invasion.
We consider that the multidisciplinary team and all-in-one centers can improve the patients’ outcome. Bringing together all the qualified members on the medical team allows to have a panoramic view on the women’s treatment path. Corroborating clinical information, ultrasound, CT, MRI, histopathology, surgical aspects, and oncological approach is in the beneficence of the patient with gynecological oncologic pathology(35). Managing the potential complication that derive from the ability of the malignant disease to metastasize(36), the side effects of aggressive chemotherapy(37), the impact of extensive surgical dissection and subsequent lymphedema and maintaining the compliance of the women are all objectives of an patient-centered healthcare system.
Conclusions
Our study presents a six-year retrospective analysis that included the clinical staging, lymph node imaging evaluation, histopathological particularities, and surgical staging. The data obtained is consistent with the current literature. The analysis reveales the modification in addressability during the COVID-19 pandemic and the change in management approach. The study of the dissection of the lymph nodes in early stages shows that an increased number of lymph nodes does not associate with an increased pathologic finding of metastatic lymph nodes.
Conflict of interest: none declared
Financial support: none declared
This work is permanently accessible online free of charge and published under the CC-BY.

Bibliografie
-
Salib MY, Russell JHB, Stewart VR, Sudderuddin SA, Barwick TD, Rockall AG, Bharwani N. 2018 FIGO Staging Classification for Cervical Cancer: Added Benefits of Imaging. Radiographics. 2020;40(6):1807-22.
-
Pecorelli S, Zigliani L, Odicino F. Revised FIGO staging for carcinoma of the cervix. Int J Gynaecol Obstet. 2009;105(2):107-8.
-
Pálsdóttir K, Fischerova D, Franchi D, Testa A, Di Legge A, Epstein E. Preoperative prediction of lymph node metastasis and deep stromal invasion in women with invasive cervical cancer: prospective multicenter study using 2D and 3D ultrasound. Ultrasound Obstet Gynecol. 2015;45(4):470-5.
-
Bhatla N, Berek JS, Cuello Fredes M, et al. Revised FIGO staging for carcinoma of the cervix uteri. Int J Gynaecol Obstet, 2019;145(1):129–35. [Published correction appears in: Int J Gynaecol Obstet. 2019;147(2):279–80.]
-
Liu MT, Hsu JC, Liu WS, Wang AY, Huang WT, Chang TH, Pi CP, HuangCY, Huang CC, Chou PH, Chen TH. Prognostic factors affecting the outcome of early cervical cancer treated with radical hysterectomy and post-operative adjuvant therapy. Eur J Cancer Care (Engl). 2008;17(2):174-81.
-
Marnitz S, Tsunoda AT, Martus P, Vieira M, Affonso Junior RJ, Nunes J, Budach V, Hertel H, Mustea A, Sehouli J, Scharf JP, Ulrich U, Ebert A, Piwonski I, Kohler C. Surgical versus clinical staging prior to primary chemoradiation in patients with cervical cancer FIGO stages IIB-IVA: oncologic results of a prospective randomized international multicenter (Uterus-11) intergroup study. Int J Gynecol Cancer. 2020;30(12):1855-61.
-
Biewenga P, van der Velden J, Mol BW, Stalpers LJ, Schilthuis MS, van der Steeg JW, Burger MP, Buist MR. Prognostic model for survival in patients with earlystage cervical cancer. Cancer. 2011;117(4):768-76.
-
Ramirez PT, Jhingran A, Macapinlac HA, Euscher ED, Munsell MF, Coleman RL, Soliman PT, Schmeler KM, Frumovitz M, Ramondetta LM. Laparoscopic extraperitoneal para-aortic lymphadenectomy in locally advanced cervical cancer: a prospective correlation of surgical findings with positron emission tomography/computed tomography findings. Cancer. 2011;117(9):1928-34.
-
Hwang L, Bailey A, Lea J, Albuquerque K. Para-aortic nodal metastases in cervical cancer: a blind spot in the International Federation of Gynecology and Obstetrics staging system: current diagnosis and management. Future Oncol. 2015;11(2):309-22.
-
Oh J, Seol KH, Choi YS, Lee JW, Bae JY. Clinical significance of lymph node size in locally advanced cervical cancer treated with concurrent chemoradiotherapy. Yeungnam Univ J Med. 2019;36(2):115-23.
-
Vázquez-Vicente D, Fernández Del Bas B, García Villayzán J, Di Fiore HA, Luna Tirado J, Casado Echarren V, García-Foncillas J, Plaza Arranz J, Chiva L. Laparoscopic paraaortic surgical staging in locally advanced cervical cancer: a single-center experience. Clin Transl Oncol. 2018;20(11):1455-9.
-
Vergote I, Pouseele B, van Gorp T, et al. Robotic retroperitoneal lower para-aortic lymphadenectomy in cervical carcinoma: first report on the technique used in 5 patients. Acta Ostet Gynecol Scand. 2008;87(7):783–7.
-
Querleu D, Dargent D, Ansquer Y, et al. Extraperitoneal endosurgical aortic and common iliac dissection in the staging of bulky or advanced cervical carcinomas. Cancer. 2000;88(8):1883–91.
-
Tsunoda AT, Marnitz S, Soares Nunes J, Mattos de Cunha Andrade CE, Scapulatempo Neto C, Blohmer JU, Herrmann J, Kerr LM, Martus P, Schneider A, Favero G, Köhler C. Incidence of histologically proven pelvic and para-aortic lymph node metastases and rate of upstaging in patients with locally advanced cervical cancer: results of a prospective randomized trial. Oncology. 2017;92(4):213-20.
-
Uzan C, Souadka A, Gouy S, et al. Analysis of morbidity and clinical implications of laparoscopic para-aortic lymphadenectomy in a continuous series of 98 patients with advanced-stage cervical cancer and negative PET-CT imaging in the para-aortic area. Oncologist. 2011;16(7):1021–7.
-
Benito V, Carballo S, Silva P, et al. Should the presence of metastatic para-aortic lymph nodes in locally advanced cervical cancer lead to more aggressive treatment strategies? J Minim Invasive Gynecol. 2017;24(4):609–16.
-
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
-
Badea M, Baroş A, Bohîlţea RE, Julea IE, Furtunescu FL, Istrate-Ofiţeru AM, Iovan L, Cîrstoiu MM, Burcin MR, Ţurcan N, Neacşu A, Berceanu C. Modern interdisciplinary monitoring of cervical cancer risk. Rom J Morphol Embryol. 2019;60(2):469-78.
-
de Gregorio A, Widschwendter P, Ebner F, et al. Influence of the New FIGO classification for cervical cancer on patient survival: a retrospective analysis of 265 histologically confirmed cases with FIGO stages IA to IIB. Oncology. 2020;98(2):91–7.
-
Matsuo K, Machida H, Mandelbaum RS, Konishi I, Mikami M. Validation of the 2018 FIGO cervical cancer staging system. Gynecol Oncol. 2019;152(1):87–93.
-
Wright JD, Matsuo K, Huang Y, et al. Prognostic Performance of the 2018 International Federation of Gynecology and Obstetrics Cervical Cancer Staging Guidelines. Obstet Gynecol. 2019;134(1):49–57.
-
Du J, Liao X. Superficial spreading squamous cell carcinoma in situ of the cervix involving the endometrium: a rare case presentation and review of literature. Int J Clin Exp Pathol. 2019;12(11):4162-6.
-
Secoşan C, Balint O, Ilian A, Bălan L, Balulescu L, Moţoc A, Zahoi D, Grigoraş D, Pirtea L. New insights in the diagnosis of rare adenocarcinoma variants of the cervix-case report and review of literature. Healthcare (Basel). 2022;10(8):1410.
-
Strojan Fležar M, Nedelko N, Poljak M, Oštrbenk Valenčak A, Gutnik H. Stratified Mucin-Producing Intraepithelial Lesion (SMILE) of the uterine cervix: high-risk HPV genotype predominance and p40 immunophenotype. Cells. 2021;10(8):2039.
-
Park JJ, Sun D, Quade BJ, Flynn C, Sheets EE, Yang A, McKeon F, Crum CP. Stratified mucin-producing intraepithelial lesions of the cervix. Adenosquamous or columnar cell neoplasia? Am J Surg Pathol. 2000;24(10):1414–9.
-
Onishi J, Sato Y, Sawaguchi A, Yamashita A, Maekawa K, Sameshima H, Asada Y. Stratified mucin-producing intraepithelial lesion with invasive carcinoma: 12 cases with immunohistochemical and utrastructural findings. Hum Pathol. 2016;55:174–81.
-
Fukui S, Nagasaka K, Iimura N, Kanda R, Ichinose T, Sugihara T, Hiraike H, Nakagawa S, Sasajima Y, Ayabe T. Detection of HPV RNA molecules in stratified mucin-producing intraepithelial lesion (SMILE) with concurrent cervical intraepithelial lesion: a case report. Virol J. 2019;16:76.
-
Lai G, Rockall AG. Lymph node imaging in gynecologic malignancy. Semin Ultrasound CT MR. 2010;31(5):363–76.
-
Yang WT, Lam WWM, Yu MY, Cheung TH, Metreweli C. Comparison of dynamic helical CT and dynamic MR imaging in the evaluation of pelvic lymph nodes in cervical carcinoma. Am J Roentgenol. 2000;175(3):759–66.
-
Pannu HK, Corl FM, Fishman EK. CT evaluation of cervical cancer: spectrum of disease. Radiographics. 2001;21(5):1155–68.
-
Batista TP, Bezerra AL, Martins MR, Carneiro VC. How important is the number of pelvic lymph node retrieved to locorregional staging of cervix cancer? Einstein (Sao Paulo). 2013;11(4):451-5.
-
Novaković P, Mandić A, Vujkov T, Tesi M, Rajović J, Zivaljević M, et al. Radical hysterectomy for stage IB1 cervical carcinoma: lymph node metastasis as a prognostic factor. J Buon. 2002;7(3):247–50.
-
Morice P, Castaigne D, Pautier P, Rey A, Haie-Meder C, Leblanc M, et al. Interest of pelvic and paraaortic lymphadenectomy in patients with stage IB and II cervical carcinoma. Gynecol Oncol. 1999;73(1):106–10.
-
Macdonald OK, Chen J, Dodson M, Lee CM, Gaffney DK. Prognostic significance of histology and positive lymph node involvement following radical hysterectomy in carcinoma of the cervix. Am J Clin Oncol. 2009;32(4):411–6.
-
Bohîlţea RE, Ţurcan G, Brătilă E, Ionescu C, Nemescu D, Bohîlţea L, Ţurcan N, Cîrstoiu M. EP31.05: Implementation of gynecological ultrasound examination report (software REGU) based on consensus of international specialised groups. Ultrasound Obstet Gynecol. 2017;50(51):398-8.
-
Rădulescu IM, Popescu R, Cîrstoiu MM, Cordoş I, Mischianu D, Cîrstoiu CF. Surgical treatment for pulmonary metastases in urogenital cancers. J Med Life. 2014;7(3):358-62.
-
Plotogea MN, Tănase AD, Secureanu AF, Ionescu S, Brătilă E, Berceanu C, Cîrstoiu MM, Mehedinţu C. The effect of estrogen deficiency related to aggressive chemotherapy on female urogenital tract. Ginecologia.ro. 2016;14(Suppl. 2):15.



