The purpose of this review is to draw attention to the importance of early diagnosis in cases of clinically significant fetomaternal hemorrhage, which is a rare but important condition to consider in the diagnosis of fetal distress. Unfortunately, since the signs of fetomaternal hemorrhage are nonspecific and the majority of cases occur spontaneously, without prior trauma or other condition, a high degree of suspicion from the clinician is necessary to establish the early treatment. The use of paraclinical tests, such as Kleihauer-Betke test and flow cytometry, is necessary to identify the passage of fetal erythrocytes into the maternal circulation. The determination of the most efficient and accessible method of diagnosis is imperative. The proposed diagnostic algorithm presented in this article can help standardize the diagnostic process of fetomateral hemorrhage, making it more efficient and precise.
Fetomaternal hemorrhage: literature review and algorithm of diagnosis
Hemoragia fetomaternă: review de literatură şi algoritm de diagnostic
First published: 26 mai 2023
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/Gine.40.2.2023.8049
Abstract
Rezumat
Scopul acestui review este de a atrage atenţia asupra importanţei diagnosticului precoce în cazul hemoragiei fetomaterne semnificative clinic, aceasta fiind o afecţiune rară, dar important de avut în vedere în cazul stabilirii diagnosticului afectării fetale. Din nefericire, având în vedere că semnele apărute în cadrul hemoragiilor fetomaterne sunt nespecifice, iar majoritatea cazurilor apar spontan, nefiind precedate de un traumatism sau de o altă afecţiune, este necesar un grad ridicat de suspiciune din partea clinicianului pentru stabilirea precoce a tratamentului necesar. Utilizarea testelor paraclinice, precum testul Kleihauer-Betke şi flowcitometria, este necesară pentru identificarea trecerii eritrocitelor fetale în circulaţia maternă, stabilirea celei mai eficiente şi accesibile metode fiind imperioasă. Algoritmul de diagnostic propus şi prezentat în acest articol poate ajuta la standardizarea procesului de diagnostic al hemoragiilor fetomaterne, acesta devenind mai eficient şi mai precis.
Introduction
Fetomaternal hemorrhage is a condition that affects both the fetus and the mother, characterized by a transfer of fetal blood into the maternal circulatory system. This occurs when there is a discontinuity in the placental barrier, and there are multiple possible causes implicated. The reported incidence of severe fetomaternal hemorrhage (more than 30 mL of fetal blood) is 0.23-0.3%(1). Up to 15% of fetal demise seems to be associated with massive fetomaternal hemorrhage(2). The volume of the blood loss can vary from a small, clinically insignificant amount to large amounts that can cause fetal anemia or even more serious complications like premature birth, stillbirth or neonatal death. Depending on the Rh factor of the fetus, another possible complication is represented by Rh alloimmunization, where maternal antibodies are directed against fetal red blood cells, leading to hemolysis and its associated severe consequences(3).
Etiopathogeny
1. Spontaneous fetomaternal hemorrhage
Spontaneous fetomaternal hemorrhage is defined as the transfer of fetal blood into the maternal circulation that occurs without any trauma history and in the absence of clinical or histopathological evidence of placental abruption. Bidirectional transfer of blood between the mother and the fetus can occur during normal physiological births. However, the reason for the occurrence of large amounts of fetal blood transfer in the absence of trauma remains unknown. Placental studies have shown a higher risk of high-volume hemorrhages associated with a less favorable clinical outcome for the fetus if the placenta presented villous edema, if nucleated red blood cells were found in the fetal vessels (nucleated erythrocytes can be found in fetal circulation normally, but are identified especially in growth restriction and fetal hypoxemia), and in the case of villous immaturity(4-6).
2. Fetomaternal hemorrhage due to traumatic or obstetrical factors
Although in most cases of fetomaternal hemorrhage, there are no causal factors identified, in some of them, certain contributing risk factors are highlighted. These can range from traumas suffered by the mother, such as those from car accidents or falls(7,8), to obstetrical procedures (such as antepartum umbilical cord blood sampling, external cephalic version, amniocentesis or chorionic villus biopsy) or obstetrical complications (placental abruption, placenta praevia, vasa praevia, choriocarcinoma, fetal death). Fetomaternal hemorrhage can be precipitated even by uterine contractions augmented with oxytocin(2,8,9). In terms of risks associated with the delivery method, no differences have been observed between vaginal and caesarean delivery. Similarly, there does not seem to be an increased risk of high-volume fetomaternal hemorrhage in twin pregnancies compared to singleton pregnancies(1).
Choriocarcinoma is a rare entity of the gestational trophoblastic disease (GTD), often underestimated and discovered late, in advanced stages. When this malignant tumor invades the myometrium, large amounts of fetal blood can pass into the maternal circulation, often associated with massive fetomaternal hemorrhage, as well as retroplacental hemorrhage, placental abruption, fetal hydrops, fetal growth restriction, severe neonatal anemia, fetal death and metastatic choriocarcinoma in both the mother and the newborn. Most of the time, the diagnosis of choriocarcinoma is secondary to perinatal complications or is determined postpartum due to maternal metastatic clinical manifestations (more frequently) or in the newborn. Metastatic disease occurring in newborns is extremely rare but, unfortunately, it is associated with a survival rate following treatment of only 17-20%. Recent data estimate the incidence of choriocarcinoma to be 1 in 160,000 births but, in reality, the number may be higher given that postpartum analysis of all the placentas is not done as a routine. Risk factors for this condition include multiparity, maternal age below 15 or above 45 years old, as well as a history of hydatidiform mole in previous pregnancies(9-12).
Diagnosis of fetomaternal hemorrhage
For a quick and efficient diagnosis of fetomaternal hemorrhage, quantitative and qualitative paraclinical tests are necessary. These tests are essential for establishing the management of this condition.
1. The Rosette test is a highly sensitive qualitative test that can detect a transfer of minimum 10 mL of fetal blood into the maternal circulation. A negative result allows for the administration of a single vial (300 µg) of Rh Ig to prevent alloimmunization in 99% of patients. In the presence of a positive result, equivalent to a fetomaternal hemorrhage of at least 10 mL, quantification is necessary either through the Kleihauer-Betke (KB) test or by flowcytometry to determine the required dose of Rh Ig(13).
2. Kleihauer-Betke test – this method has been used for over six decades to quantitatively determine fetomaternal hemorrhage, with the primary purpose to prevent efficiently the Rh (D) alloimmunization and to improve the fetal monitoring in case of fetomaternal hemorrhage(14). It still remains the most commonly used diagnostic method because it is inexpensive and does not require special equipment or specialized training of medical personnel(3,14). The principle underlying this analysis is represented by the lower susceptibility of fetal erythrocytes to acid elution, given the predominance of HbF, as opposed to adult erythrocytes, which are acid-sensitive due to the presence of adult hemoglobin. However, this test also has some drawbacks. In addition to being very laborious (counting at least 2000 erythrocytes is recommended), the precision and accuracy of the test may be suboptimal due to a lack of standardization, which can lead to variations in the characteristic of the procedure (density of the blood smear, variations in the pH of the buffer system used), variations in the interpretation of results by different observers or variations between different centers etc.(13,16) (according to the study conducted by Duckett and Constantine, there are significant differences in the results obtained through the KB test, with the error rate in identifying the actual number of fetal erythrocytes being over 500% among observers from the same medical center using a test with the same execution characteristics; moreover, the error rate of interhospital results is even higher(15)). False positive results may also occur within the KB test, as it is based on visual discrimination between fetal and adult erythrocytes (dark pink for fetal cells and minimally colored for adult cells), through the presence of F cells (erythrocytes with intermediate concentrations of HbF – 20-25%, present in increased quantities in inherited hemoglobinopathies such as sickle cell anemia, beta-thalassemia, but also in acute stress erythropoiesis and pregnancy) or it may lead to an overestimation of the magnitude of a true fetomaternal hemorrhage(13).
3. Flowcitometry – currently, many medical centers have adopted the use of flowcytometry as a routine for the quantification of fetomaternal hemorrhage. This test represents a quantitative method in which fetal red blood cells (HbF) bound to monoclonal antibodies are counted and measured by fluorescence intensity. The advantages of flowcytometry over the KB test include increased precision, reduced processing time, as it is an automated process (a larger number of red blood cells can be analyzed, respectively more than 50,000), and the high reproducibility of the obtained results(4,13,14). The main limitation of flowcytometry is the higher cost, which is why it is not available in all centers(14). It has been observed that, in the case of pregnancies before term, a flowcytometry result above 1% was always associated with clinically significant fetomaternal hemorrhage, while in the case of term pregnancies (above 37 gestational weeks), a flowcytometry result above 5% was needed for the hemorrhage to be clinically significant. The explanation for this finding is the increase in fetal blood volume as the pregnancy advances. In the case of an abnormal flowcytometry result in association with a normal clinical picture of the newborn, the most likely explanation is the presence of multiple repeated episodes of antepartum fetomaternal hemorrhage compensated by the fetus(8).
4. High-performance liquid chromatography – as the number of pregnant women with hemoglobinopathies has increased over time, this test has mostly been abandoned(9).
Diagnosis of fetal distress
The early identification of the signs of fetomaternal hemorrhage is essential to prevent fetal and maternal distress. In the cases of clinically significant fetomaternal hemorrhage, fetal distress can be identified, but establishing a prenatal diagnosis of severe fetomaternal hemorrhage based on these changes is difficult(17).
The earliest sign of fetal distress is represented by the decrease or the absence of fetal movements, perceived or not by the mother. This is a nonspecific sign, but it should be taken as a warning for clinicians and it should determine them to perform additional tests to improve the accuracy of fetal status assessment(4,16,18).
The next most commonly encountered alteration is represented by the increase of peak velocity of the blood in the medial cerebral artery (MCA) of the newborn(17-19). A Doppler value of this parameter higher than 1.5 MoM represents a reliable sign of moderate to severe fetal anemia(20). There are plenty of causes of fetal anemia, with the most common being alloimmunization and infections, parvovirus B19 being the most frequently involved. Other possible causes include arteriovenous malformations of the fetus or the placenta, vascular tumors, fetal aneuploidies and fetomaternal hemorrhages that can occur as an acute event or persist as a chronic hemorrhage. In the case of acute anemia, the modification of peak velocity in MCA is often the only ultrasound sign found, while in the case of chronic anemia, signs of hydrops and serous effusion may also occur(18,20,21).
There have also been reported changes in the fetal heart rate monitoring (CTG) as a result of fetomaternal hemorrhage. There is no specific value of fetal blood volume lost that determines the appearance of CTG to change, but a clinically significant fetomaternal hemorrhage will lead to the recording of nonspecific deceleration patterns, absent accelerations, and fetal tachycardia through the activation of the fetal compensatory adrenergic response. The recording of a sinusoidal fetal heart rate pattern is suggestive of the presence of fetal anemia(4,17,22). However, this pattern can be misinterpreted and it is often intercalated with a pseudosinusoidal pattern that frequently occurs during normal births and occasionally during pregnancy, being associated with a good fetal prognosis(9). Another change found on the CTG that may raise suspicion of fetal anemia is the presence of a sawtooth pattern, which can appear in both fetomaternal hemorrhage and fetal hypotension(4).
The following diagnostic algorithm (Figure 1) may help in the standardization of the diagnostic protocol of fetomaternal hemorrhage, making it more efficient and precise.
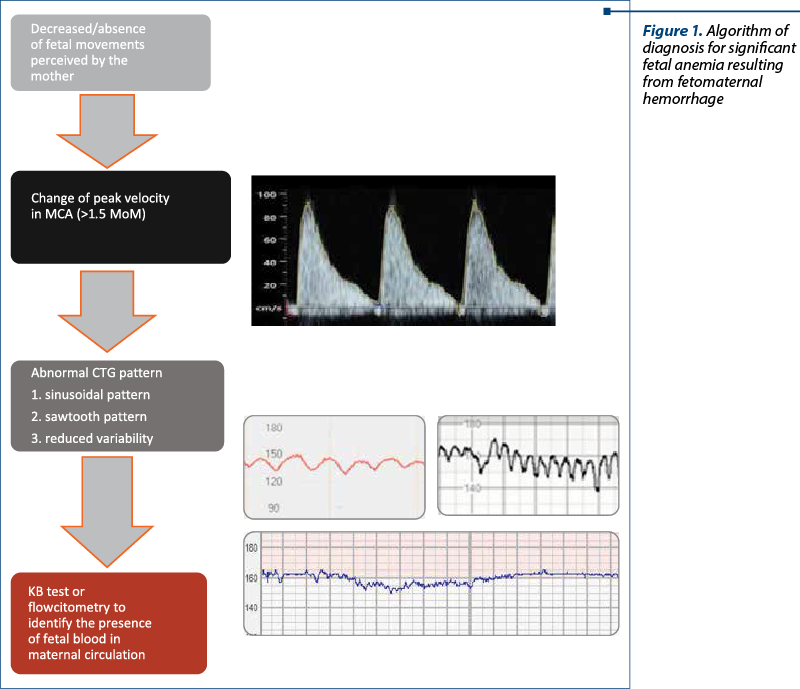
A case report by Gică et al., published in 2021, suggestive for the necessity of a diagnostic algorithm, presents a case of spontaneous fetomaternal hemorrhage, involving a 32-year-old para I, Rh-positive woman with 37 weeks and 2 days of gestation. There were no complications during the pregnancy until the time of hospitalization. The reason for coming to the hospital was represented by decreased fetal movements. The physical examination did not reveal any pathological signs. Also, after using ultrasound examination, the only pathological sign was the peak systolic velocity change in the MCA>1.5 MoM. CTG recording showed the presence of tachycardia, reduced variability and decelerations, suggestive of chronic fetal cerebral hypoxia. The chosen method of treatment was the child birth through caesarean section due to severe fetal anemia with non-reassuring CTG, resulting in the extraction of a male newborn with an Apgar score of 4 at 1 minute and 6 at 5 minutes, requiring intense resuscitation for hypovolemia. Fetal blood analysis revealed an increased number of reticulocytes, suggesting that the hemorrhage occurred 1-2 days before the delivery(4).
Regarding the clinical manifestations of the mother, fetomaternal hemorrhage is usually clinically silent. Sometimes, however, clinical signs suggestive for a blood transfusion may occur (fever, shiver or nausea)(4).
Unexplained neonatal anemia along with increased production of red blood cell precursors confirm the massive peripartum fetomaternal hemorrhage.
Management
Once the suspicion of fetal anemia caused by fetomaternal hemorrhage is established, the doctors have to decide upon the decision of treatment. The decisive factor for choosing the treatment method is based on the gestational age at the time of diagnosis. In cases of term or near-term pregnancies (>34 weeks of gestation), the main indication is delivery. In cases of pregnancies with lower gestational ages (<32 weeks of gestation), the patient should be admitted to a tertiary maternity hospital for evaluation and treatment, which may include intrauterine transfusion, allowing the pregnancy to continue until it reaches a gestational age safe for delivery. In cases of pregnancies with gestational ages between 32 and 34 weeks, the risks associated with intrauterine transfusion need to be weighed against the risks associated with prematurity/neonatal transfusion. Due to the limited number of cases of fetomaternal hemorrhage, the effect of intrauterine transfusion on the long-term prognosis of neonates is not known(4,9,19,23).
Intrauterine transfusion is a relatively safe procedure, with a risk of pregnancy loss of 1.5-3%(24). Fetal distress may occur as a result of acute complications of the procedure (umbilical cord complications – rupture, spasm; tamponade due to a hematoma, excessive bleeding from the puncture site; volume overload; chorioamnionitis; premature rupture of membranes; premature labor), which can further complicate with fetal or neonatal death, premature birth, or neonatal asphyxia. Additionally, long-term complications may arise, such as the need for additional erythrocyte transfusions during the first six months of life, explained by the fetal erythropoiesis suppression. Erythrocyte transfusions present a minimal risk but, theoretically, anaphylactic reactions and viral disease transmission can occur. Furthermore, the administration of blood transfusions may induce the production of antierythrocyte antibodies that can complicate future transfusions by inducing delayed hemolytic transfusion reactions(23).
Prognosis
The prognosis of fetomaternal hemorrhage largely depends on the volume of fetal blood transfused into the maternal circulation. The loss of a blood volume higher than 20 ml/kg is directly proportional to the number and severity of fetal complications, including premature birth, transfer to the neonatal intensive care unit, neonatal anemia requiring blood transfusion, and even fetal death in utero(2).
Although some studies, such as the one by Rubod et al.(2), report that no neurological sequelae attributable to fetomaternal hemorrhage have been identified in children followed-up in the long term, in the study conducted by Kadooka et al.(25), out of the 18 patients studied in the long term, nine had an unfavorable prognosis with neurological impairment – four patients with cerebral palsy and five patients, each with one of the following: mental retardation; mental retardation and cerebral palsy; cerebral palsy and ADHD; cerebral palsy and epilepsy; cerebral palsy, mental retardation and epilepsy.
Fortunately, the risk of recurrence of fetomaternal hemorrhage in subsequent pregnancies is low, and clinicians can reassure patients about this aspect. However, a high degree of suspicion is necessary in cases of unexplained fetal death in order to diagnose any fetal involvement early and to perform the necessary tests in a timely manner(8).
Indeed, estimating the recurrence rate of fetomaternal hemorrhage poses a challenge due to the incomplete reporting of cases. Additionally, the analysis of fetal hemoglobin (HbF) by flowcytometry is not performed as a routine in all medical centers, which may lead to underestimating the actual recurrence rate(8).
Alloimmunization as a result of fetomaternal hemorrhage
In the case of Rh-negative mothers and Rh-positive fetuses, the interruption of the placental barrier followed by the passage of fetal blood into the maternal circulation leads to sensitization of the maternal immune system with the formation of anti-D antibodies directed against fetal red blood cells. IgG antibodies are implicated in the development of hemolytic disease(13,26,27). Hemolytic disease of the fetus and newborn (HDFN) is a condition which, if left untreated, can lead to increased perinatal mortality and substantial morbidity, with the risk of long-term organic and functional disorders. The consequences of HDFN include fetal anemia, cardiomegaly due to hyperdynamic circulation, and fetal hydrops. Another serious complication is the accumulation of bilirubin following the hemolysis of fetal red blood cells. Elevated bilirubin levels lead to fetal nervous system impairment, with the appearance of nuclear jaundice (kernicterus), characterized by cerebral palsy, hearing loss or deafness and psychomotor handicaps(3). Prevention of Rh D alloimmunization is achieved through the administration of Rh immunoglobulin. Following the introduction of prophylaxis with anti-D Ig administered at 28 gestational weeks and at birth in Rh-negative mothers with Rh-positive fetuses, the incidence of alloimmunization has decreased from 16% to 0.07%(1). A dose of 300 µg of anti-D Ig is capable of neutralizing 30 ml of fetal blood or 15 mL of fetal red blood cells that have entered the maternal circulation. Given that transplacental hemorrhage with a quantity of fetal red blood cells higher than 1 ml is unlikely, it is considered that a dose of anti-D Ig administered at 28 gestational weeks is sufficient for antenatal prophylaxis. However, during birth or spontaneous massive fetomaternal hemorrhage, the passage can exceed 30 mL of fetal blood, necessitating an appropriate dosage of anti-D Ig(1,13).
Conclusions
Fetomaternal hemorrhage is a rare but potentially dangerous condition for both the baby and the mother. For the diagnosis of this condition and for the initiation of prompt and optimal treatment, a higher degree of clinical suspicion is required to perform the necessary diagnostic tests, as the clinical signs are nonspecific. New studies are needed for a better understanding of the pathophysiology of this process, for identifying the most efficient methods of diagnosis and treatment, and for establishing the long-term prognosis following fetomaternal hemorrhage and delivery, as well as for fetomaternal hemorrhage followed by intrauterine transfusions.
The diagnostic algorithm presented in this article can be useful for clinicians, primarily by organizing the process of determining the cause of fetal impairment. It can also help to increase the precision and efficiency of the diagnostic process, thereby reducing costs and the time required to reach a diagnosis.
Conflict of interest: none declared
Financial support: none declared
This work is permanently accessible online free of charge and published under the CC-BY.

Bibliografie
-
Salim R, Ben-Shlomo I, Nachum Z, Mader R, Shalev E. The incidence of large fetomaternal hemorrhage and the Kleihauer-Betke test. Obstet Gynecol. 2005;105(5 Pt 1):1039-44.
-
Rubod C, Deruelle P, Le Goueff F, Tunez V, Fournier M, Subtil D. Long-term prognosis for infants after massive fetomaternal hemorrhage. Obstet Gynecol. 2007;110(2 Pt 1):256-60.
-
de Haas M, Thurik FF, Koelewijn JM, van der Schoot CE. Haemolytic disease of the fetus and newborn. Vox Sang. 2015;109(2):99-113.
-
Gică N, Botezatu R, Demetrian M, et al. Severe neonatal anemia due to spontaneous massive fetomaternal hemorrhage at term: an illustrative case with suspected antenatal diagnosis and brief review of current knowledge. Medicina (Kaunas). 2021;57(12):1285.
-
Ilagan NB, Elias EG, Liang KC, Kazzi G, Piligian J, Khatib G. Perinatal and neonatal significance of bacteria-related placental villous edema. Acta Obstet Gynecol Scand. 1990;69(4):287-90.
-
Blog Comments. ITNOW. 2011;53(2):34.
-
Lipitz S, Achiron R, Horoshovski D, Rotstein Z, Sherman D, Schiff E. Fetomaternal haemorrhage discovered after trauma and treated by fetal intravascular transfusion. Eur J Obstet Gynecol Reprod Biol. 1997;71(1):21-2.
-
Boller MJ, Moore GS, Hung YY, Ritterman Weintraub ML, Schauer GM. Fetomaternal hemorrhage: evaluation of recurrence within a large integrated healthcare system. Am J Obstet Gynecol. 2021;225(5):540.e1-540.e8.
-
Stefanovic V. Fetomaternal hemorrhage complicated pregnancy: risks, identification, and management. Curr Opin Obstet Gynecol. 2016;28(2):86-94.
-
Simões M, Vale G, Lacerda C, Pais P, Pignatelli D. Fetomaternal hemorrhage: a clue to intraplacental choriocarcinoma and neonatal malignancy. J Matern Fetal Neonatal Med. 2022;35(25):6615-7.
-
Liu J, Guo L. Intraplacental choriocarcinoma in a term placenta with both maternal and infantile metastases: a case report and review of the literature. Gynecol Oncol. 2006;103(3):1147-51.
-
Sebire NJ, Lindsay I, Fisher RA, Seckl MJ. Intraplacental choriocarcinoma: experience from a tertiary referral center and relationship with infantile choriocarcinoma. Fetal Pediatr Pathol. 2005;24(1):21-9.
-
Kim YA, Makar RS. Detection of fetomaternal hemorrhage. Am J Hematol. 2012;87(4):417-23.
-
Stanic Z, Stanisic L, Fureš R, Persec Z, Dapic K, Hrgovic Z. Fetomaternal hemorrhage: can we use hemoglobin electrophoresis as a diagnostic tool?. Z Geburtshilfe Neonatol. 2020;224(3):150-2.
-
Duckett JR, Constantine G. The Kleihauer technique: an accurate method of quantifying fetomaternal haemorrhage?. Br J Obstet Gynaecol. 1997;104(7):845-6.
-
Tao E, Ye D, Long G, et al. Severe neonatal anemia affected by massive fetomaternal hemorrhage: a single-center retrospective observational study. J Matern Fetal Neonatal Med. 2022;35(20):3972-8.
-
Bellussi F, Perolo A, Ghi T, Youssef A, Pilu G, Simonazzi G. Diagnosis of severe fetomaternal hemorrhage with fetal cerebral doppler: case series and systematic review. Fetal Diagn Ther. 2017;41(1):1-7.
-
Schmit M, Duminil L, Loron G, Bednarek N, Graesslin O, Raimond E. Massive feto-maternal hemorrhage: Two cases. J Gynecol Obstet Hum Reprod. 2019;48(7):533-5.
-
Sueters M, Arabin B, Oepkes D. Doppler sonography for predicting fetal anemia caused by massive fetomaternal hemorrhage. Ultrasound Obstet Gynecol. 2003;22(2):186-9.
-
Mari G, Deter RL, Carpenter RL, et al. Noninvasive diagnosis by Doppler ultrasonography of fetal anemia due to maternal red-cell alloimmunization. Collaborative Group for Doppler Assessment of the Blood Velocity in Anemic Fetuses. N Engl J Med. 2000;342(1):9-14.
-
Prefumo F, Fichera A, Fratelli N, Sartori E. Fetal anemia: Diagnosis and management. Best Pract Res Clin Obstet Gynaecol. 2019;58:2-14.
-
Haruna Y, Suzuki S. Cardiotocography findings of early-stage chronic fetomaternal hemorrhage after the presentation of reduced fetal movement. Clin Case Rep. 2019;7(3):564-7.
-
Lindenburg IT, van Kamp IL, Oepkes D. Intrauterine blood transfusion: current indications and associated risks. Fetal Diagn Ther. 2014;36(4):263-71.
-
Levy-Zauberman Y, Mailloux A, Kane A, Castaigne V, Cortey A, Carbonne B. Massive fetomaternal hemorrhage secondary to intrauterine intravascular transfusion. Obstet Gynecol. 2011;118(2 Pt 2):439-42.
-
Kadooka M, Kato H, Kato A, Ibara S, Minakami H, Maruyama Y. Effect of neonatal hemoglobin concentration on long-term outcome of infants affected by fetomaternal hemorrhage. Early Hum Dev. 2014;90(9):431-4.
-
Cardoso MR, de Souza-Araújo CN, Talarico MCR, Heinrich-Mouçouçah J, Guimarães F, Barini R. Evaluation of automatic blood analyzer as screening method in fetomaternal hemorrhage. Biomed Res Int. 2019;2019:6481654.
-
Myle AK, Al-Khattabi GH. Hemolytic disease of the newborn: a review of current trends and prospects. Pediatric Health Med Ther. 2021;12:491-8.