From genes to clinical practice – the role of immunohistochemistry in the classification of breast carcinomas
De la determinismul genetic la abordarea clinică – rolul imunohistochimiei în clasificarea carcinoamelor mamare
Abstract
Breast cancer is a complex disease that can be caused by a combination of genetic and environmental factors. Several genes have been identified to be associated with an increased risk of breast cancer, including BRCA1, BRCA2, TP53, PTEN and others. These genes can be tested for mutations in order to assess an individual’s risk of developing breast cancer. Immunohistochemistry (IHC) plays a significant role in personalized breast cancer treatment by providing information on the specific proteins and biomarkers present in a patient’s tumor. This information can help guide treatment decisions and improve outcomes. Immunohistochemistry is used to determine the expression levels of several proteins, including estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2). ER and PR are hormone receptors that are commonly expressed in breast cancer cells. Their expression levels are important for determining treatment options, as hormone therapy can be effective in patients whose tumors express these receptors. HER2, on the other hand, is a growth factor receptor which is overexpressed in some breast cancers. HER2-positive tumors can be treated with targeted therapy drugs such as trastuzumab. In addition to ER, PR and HER2, IHC can also be used to assess the expression of other markers in breast cancer, such as Ki-67, a marker of cellular proliferation, and cytokeratins, which are intermediate filaments found in epithelial cells. This information can help determine the aggressiveness of the tumor and the likelihood of response to certain treatments. Overall, the identification of breast cancer genes and the use of immunohistochemistry to assess protein expression levels are important tools for the diagnosis and treatment of breast cancer.Keywords
breast cancerHer2-positiveluminal Aluminal Bsubtypes of breast cancerbasal-likeRezumat
Cancerul mamar este o boală complexă care poate fi cauzată de o combinaţie de factori genetici şi de mediu. Au fost identificate mai multe gene care sunt asociate cu un risc crescut de cancer de sân, inclusiv BRCA1, BRCA2, TP53, PTEN şi altele. Aceste gene pot fi testate pentru mutaţii cu scopul de a evalua riscul unei persoane de a dezvolta cancer de sân. Imunohistochimia (IHC) joacă un rol important în tratamentul personalizat al cancerului de sân, prin furnizarea de informaţii cu privire la proteinele specifice şi biomarkerii tumorali. Aceste informaţii pot ajuta la ghidarea deciziilor de tratament şi la îmbunătăţirea prognosticului pacientelor. Imunohistochimia este utilizată pentru a determina nivelurile de expresie ale mai multor proteine, inclusiv receptorul de estrogen (ER), receptorul de progesteron (PR) şi receptorul factorului de creştere epidermal uman 2 (HER2). ER şi PR sunt receptori hormonali care sunt frecvent exprimaţi în celulele cancerului de sân. Nivelurile lor de expresie sunt importante pentru determinarea opţiunilor de tratament, deoarece terapia hormonală poate fi eficientă la pacienţii ale căror tumori exprimă aceşti receptori. HER2 este un receptor al factorului de creştere care poate fi supraexprimat în unele tipuri de cancer mamar. Tumorile HER2-pozitive pot fi tratate cu medicamente terapeutice specifice, cum ar fi trastuzumab. Pe lângă evaluarea expresiei ER, PR şi HER2, IHC poate fi de asemenea utilizată pentru a evalua expresia altor markeri în cancerul de sân, cum ar fi Ki-67, un marker al proliferării celulare, şi citokeratinele, care sunt filamente intermediare găsite în celulele epiteliale. Aceste informaţii pot ajuta la determinarea agresivităţii tumorii şi a probabilităţii de răspuns la anumite tratamente. În concluzie, identificarea genelor cancerului de sân şi utilizarea imunohistochimiei pentru a evalua nivelurile de expresie ale proteinelor sunt instrumente importante pentru diagnosticarea şi tratamentul cancerului de sân.Cuvinte Cheie
cancer mamarHer2 pozitivluminal Aluminal Bsubtipuri de cancer mamarbasal-likeTumor biology, in addition to tumor size, lymph node status and metastasis presence, is definitely important for prognosis and response to therapy. From a histological and molecular perspective, breast carcinomas are heterogeneous – different gene expression patterns are responsible for the wide range of variations in their prognosis and therapeutic response. The variety of genetic abnormalities in breast carcinomas has been emphasized in several studies over the past few decades, enabling the investigation of the gene expression profile (GEP) and immunohistochemistry subtyping of breast cancer(1).
Breast tumor identification and segregation into intrinsic molecular subtypes has enabled much more efficient clinical management, using personalized therapies, and the assessment of recurrence risk based on the molecular characteristics of breast neoplasms. In fact, the term breast cancer encompasses a variety of conditions, some of which are comparable from a histology standpoint but distinct from one another in terms of biology and therapeutic response. The management of all cases should be based on clinical and imaging data, together with histological and molecular studies (Figure 1).
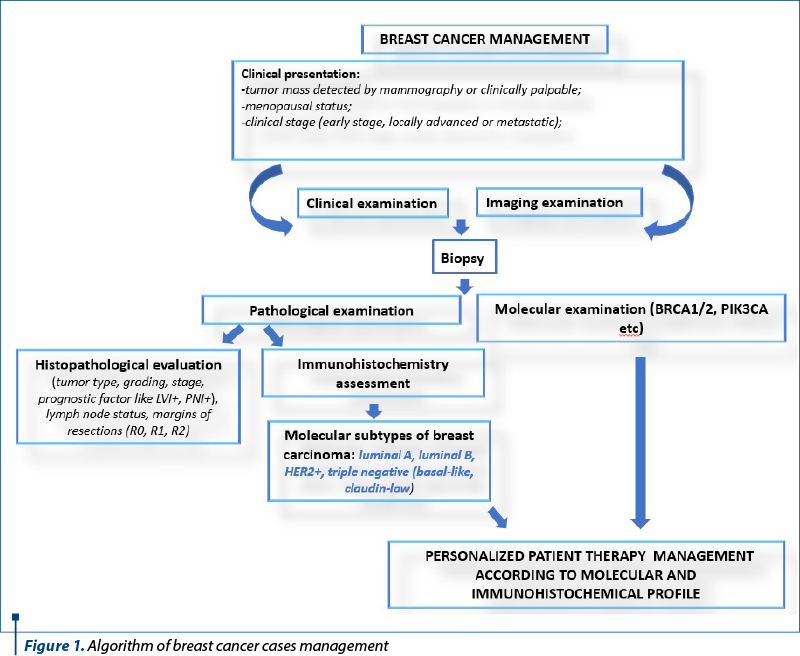
In 2013, the St. Gallen International Breast Carcinoma Conference defined five molecular subtypes of breast cancer: luminal A and B, HER2, basal-like and claudin-low(2).
The treatment was initially surgical, with or without radiation therapy, based on grade, size, nodal status and metastasis, and nowadays it includes the evaluation of sentinel lymph nodes, the evaluation of biomarkers and genetic profiling.
Multiple studies have validated this classification, which initially emphasized the division of all breast carcinomas into estrogen receptor (ER)-positive and ER-negative subtypes, and several subtypes have been added and then removed from the initial panel as new discoveries have emerged. To put this classification into practice, immunohistochemical surrogate algorithms were developed(3) (Figure 2).
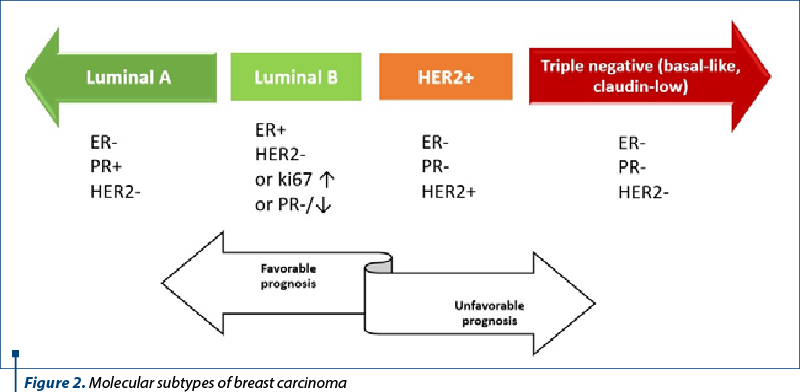
Breast carcinomas that express hormone receptors like estrogen and progesterone will benefit from endocrine therapy. Another hormone receptor – androgen – is considered to have predictive value, and might be part of the classification in the future.
Breast carcinomas in the luminal molecular subtype category account for 60-70% of all breast carcinomas and have a better prognosis than breast neoplasms with negative hormone receptors. They are divided into two molecular subtypes: A and B.
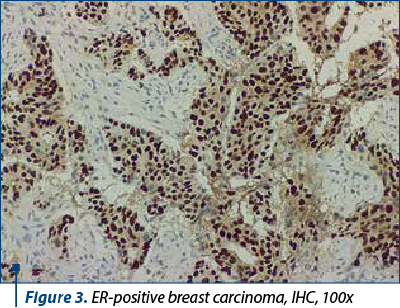
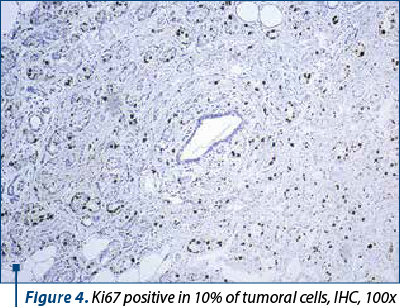
Around 40-60% of breast tumors are molecular subtype A (luminal A-like) breast carcinomas, which are distinguished by low histological grade, positive hormone receptors (ER/PR+) (Figure 3), negative HER2, and a low Ki67 proliferation index (Figure 4). This molecular subtype of breast cancer has poorer rates of response to chemotherapy, but responds well to hormone treatment(4).
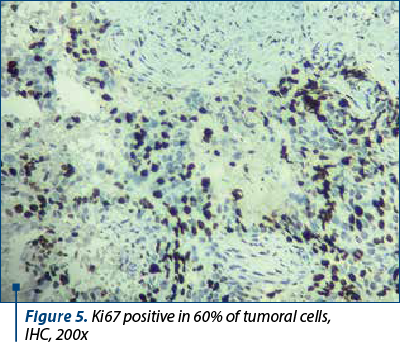
About 20-30% of breast tumors are breast carcinomas of molecular subtype B, or luminal B-like, which have less differentiated histological grades, higher recurrence rates, lower expression of estrogen and progesterone hormone receptors, variable expression of the HER2 protein, and higher proliferative rates (Ki67 proliferation index) – Figure 5. Compared to molecular subtype A, molecular subtype B is associated with a worse prognosis and with a better response to chemotherapy(5).
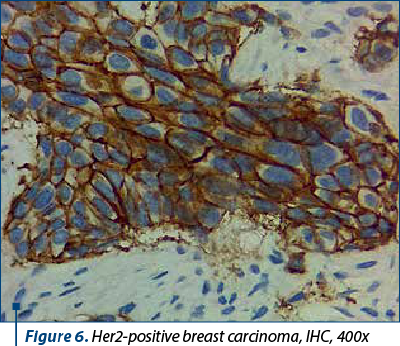
Between 10% and 20% of breast carcinomas fall under the HER2 molecular subtype, which is identified by the overexpression of the Her2 neu protein (Figure 6) and the lack of hormone receptor expression. It is distinguished by clinically aggressive breast cancers, high histological grade, a poorer long-term prognosis brought by early-onset systemic metastases, and worse survival rates. IHC and ISH can detect HER2 overexpression, but good clinical practice in the pathology laboratory is required because many variables can interfere with the assessment(6). Although immunohistochemistry is still the most frequently used initial test and the primary technique in practice, its effectiveness is highly dependent on the pre- and post-analytical conditions.
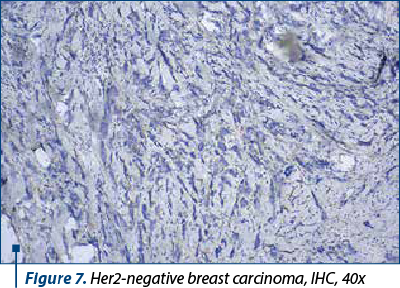
The molecular basal-like breast cancer subtype accounts for about 15-20% of breast carcinomas and is distinguished by the absence of hormonal receptor expression (ER-, PR-, HER2-) (Figure 7), as well as immunohistochemical positivity for cytokeratin 5/6 (CK5/6) and 14 (CK14) and/or EGFR (HER1). It is linked to increased rates of genomic instability, proliferation and histological grade, as well as a higher prevalence of BRCA1 mutations(7). Basal-like 1 (BL-1), basal-like 2 (BL-2), mesenchymal and luminal AR, with various survival rates and a variable level of responsiveness to neoadjuvant therapy, are the four subtypes that have been identified based on gene expression(8).
HER2, estrogen, progesterone, claudin 3, claudin 4, claudin 7 and E-cadherin immunohistochemistry expression are not present in the 5% of breast carcinomas that belong to the claudin-low breast subtype (claudin levels are reduced in these cancers). It is linked to elevated gene expression for immune system and inflammation-related genes, which supports the development of a significant lymphocytic chronic inflammatory infiltrate(9).
HER2-low breast cancer is a new emerging subtype with clinical significance. It includes tumors with lower levels of the HER2 protein on their surface and with IHC scores of 1+ and 2+ without amplification, but there is no specific definition or set of criteria that would characterize a tumor as low HER2, because low levels are not yet classified within ASCO 2018(10).
Breast cancer, like other cancers, is not a single disease. The cancers vary tremendously, not only in histologic appearance, grade, hormone receptor and HER2 status, but also on a molecular basis. There is no longer a question of morphology versus molecular debate. Personalized treatment is based on a pathology report that combines traditional morphology and immunohistochemistry with a variety of molecular tests. To remain clinically relevant, it is critical to change the staging as new discoveries in cancer research and treatment emerge.
Conflict of interest: none declared
Financial support: none declared
This work is permanently accessible online free of charge and published under the CC-BY.

Bibliografie
-
Testa U, Castelli G, Pelosi E. Breast Cancer: A molecularly heterogenous disease needing subtype-specific treatments. Med Sci (Basel). 2020;8(1):18.
-
Lüönd F, Tiede S, Christofori G. Breast cancer as an example of tumour heterogeneity and tumour cell plasticity during malignant progression. Br J Cancer. 2021;125:164–75.
-
Comănescu M. Basic molecular pathology in breast carcinoma. In: Stolnicu S, Alvarado-Cabrero I. (eds) Practical Atlas of Breast Pathology. 2018, Springer, Cham.
-
Yersal O, Barutca S. Biological subtypes of breast cancer: Prognostic and therapeutic implications. World J Clin Oncol. 2014;5(3):412-24.
-
Fragomeni SM, Sciallis A, Jeruss JS. Molecular subtypes and local-regional control of breast cancer. Surg Oncol Clin N Am. 2018;27(1):95-120.
-
Morganti S, Ivanova M, Ferraro E, Ascione L, Vivanet G, Bonizzi G, Curigliano G, Fusco N, Criscitiello C. Loss of HER2 in breast cancer: biological mechanisms and technical pitfalls. Cancer Drug Resist. 2022;5(4):971-80.
-
Cheang MC, Voduc D, Bajdik C, Leung S, McKinney S, Chia SK, Perou CM, Nielsen TO. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008;14(5):1368-76.
-
Alluri P, Newman LA. Basal-like and triple-negative breast cancers: searching for positives among many negatives. Surg Oncol Clin N Am. 2014;23(3):567-77.
-
Logullo AF, Pasini FS, Nonogaki S, Rocha RM, Soares FA, Brentani MM. Immunoexpression of claudins 4 and 7 among invasive breast carcinoma subtypes: A large diagnostic study using tissue microarray. Mol Clin Oncol. 2018;9(4):377-88.
-
Agostinetto E, Rediti M, Fimereli D, Debien V, Piccart M, Aftimos P, Sotiriou C, de Azambuja E. HER2-low breast cancer: molecular characteristics and prognosis. Cancers (Basel). 2021;13(11):2824.



