Infecţiile postoperatorii în ginecologie
Surgical site infection in gynecology
Abstract
Objective. The morbidity and mortality caused by surgical site infections (SSIs) worldwide have become a major public health issue. Furthermore, the prevention of SSI remains a focus for healthcare systems and hospitals due to its healthcare cost reducibility. The objective of this review is to assess the guidelines recommendations related to the prevention, diagnosis and management of SSIs. Materials and method. This paper is a review based on information found in literature. The analysis was limited to English language articles and guidelines published between January 1st, 2015 and April 30th, 2020, on PubMed, using the following keywords: surgical site infection, wound infection in gynecology, prophylactic antibiotics, economic impact of SSIs. In this review we discuss the current trends in the management of SSIs in gynecology. Results. The microorganisms in gynecologic surgery are unique as the site of infection may be from the vaginal/genitourinary tract, the abdomen or the skin. The most frequent bacteria in abdominal SSI in gynecologic surgery are aerobic Gram-positive cocci (Staphylococcus aureus, Staphylococcus epidermidis). Also, incisions made around the perineum and the groin can become infected with Enterococcus species and Escherichia coli. Incisional cellulitis and vaginal cuff cellulitis represent the most common superficial SSI. Deep tissue abscess represents the most common deep infection, whilst tubo-ovarian abscesses and pelvic abscesses constitute the most common space and organ infections. The most serious form of SSI is necrotizing fasciitis, a life-threatening complication. Conclusions. By rigorously following the clinical recommendations and evidence, the prevention, diagnosis and treatment of SSI can be managed correctly. As a result, health outcomes and healthcare-related costs will be greatly improved, thereby the quality of healthcare that we deliver to patients will be furthermore increased.Keywords
surgical site infection (SSI)wound infection in gynecologyprophylactic antibioticseconomic impact of SSIRezumat
Obiectiv. Morbiditatea şi mortalitatea cauzate de infecţiile postoperatorii au devenit o problemă majoră de sănătate publică în întreaga lume. Mai mult, prevenirea acestora rămâne un obiectiv al sistemelor de asistenţă medicală şi al spitalelor, în scopul reducerii costurilor de îngrijire a pacienţilor. Obiectivul acestei recenzii este de a trece în revistă ghidurile şi recomandările legate de prevenirea, diagnosticul şi gestionarea infecţiilor postoperatorii în ginecologie. Materiale şi metodă. Această lucrare este o recenzie bazată pe informaţiile din literatura de specialitate. Analiza s-a limitat la articole şi ghiduri în limba engleză, publicate între 1 ianuarie 2015 şi 30 aprilie 2020, pe PubMed, folosind următoarele cuvinte-cheie: infecţie la nivelul zonei operate, infecţia plăgilor în ginecologie, antibiotice profilactice, impactul economic al infecţiilor postoperatorii. În această revizuire a literaturii, discutăm tendinţele actuale în managementul SSI în ginecologie. Rezultate. Microorganismele din chirurgia ginecologică sunt din tractul vaginal/genito-urinar, abdomen sau piele. Cele mai frecvente infecţii în chirurgia ginecologică sunt cauzate de bacterii aerobe Gram-pozitive, respectiv coci (Staphylococcus aureus, Staphylococcus epidermidis), deşi inciziile făcute în jurul perineului şi la nivel inghinal pot fi, de asemenea, infectate cu speciile Enterococcus şi Escherichia coli. Cele mai frecvente infecţii superficiale în ginecologie sunt celulita incizională şi celulita bontului vaginal. Cea mai frecventă infecţie a ţesuturilor profunde este abcesul, iar cele mai comune infecţii ale cavităţilor şi organelor sunt abcesele tubo-ovariene şi abcesele pelviene, inclusiv abcese ale bontului vaginal. Cea mai gravă formă de infecţie este fasciita necrozantă, o complicaţie care poate pune viaţa pacientei în pericol dacă nu este diagnosticată şi tratată rapid şi adecvat. Concluzii. Respectând recomandările şi dovezile clinice, putem preveni, diagnostica şi trata în mod corect infecţiile postoperatorii. La rândul său, această conduită va îmbunătăţi rezultatele şi va scădea costurile legate de îngrijirea medicală, crescând astfel calitatea asistenţei medicale.Cuvinte Cheie
infecţie la locul chirurgicalinfecţie a plăgilor în ginecologieantibiotice profilacticeimpactul economic al infecţiilor postoperatoriiIntroduction
Worldwide, healthcare-associated infections (HAI) represent the most frequent complication affecting the safety of the patients. Most recent medical updates made by the World Health Organization (WHO) point out that surgical site infection (SSI) represents the most explored and frequent type of HAI and affects up to one-third of patients who experienced a surgical intervention. In Europe and the United States of America, SSI remains the most frequent type of HAI, although SSI incidence is lower in high-income countries(1). Globally, the morbidity and mortality caused by SSI have become a major public health issue. Furthermore, a global increase in antibiotic resistance associated with SSIs has also become a therapeutic challenge for physicians worldwide. Healthcare systems and hospitals could directly benefit from SSI prevention as it is a resoluble healthcare cost(2).
Surgical site infections
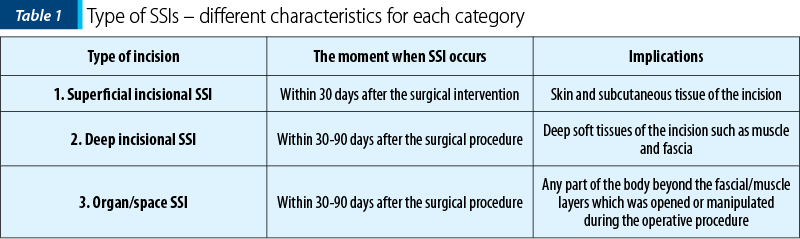
According to the US CDC National Healthcare Safety Network (CDC-NHSN), the standard definition of SSI divides SSIs into three categories: superficial incisional, deep incisional and organ/space infections. As noticed in Table 1, for each infection, different characteristics qualify them for each category(3-5).
In a report dating from 2015 about SSI prevention, multiple factors were identified which influence the development of healthcare-associated infections. A major factor is represented by the preoperative evaluation and preparation of the patient, as well as the outcome of the perioperative environment, surgeon and healthcare delivery factors on SSI prevention, as well as management concerns (Figure 1)(6).
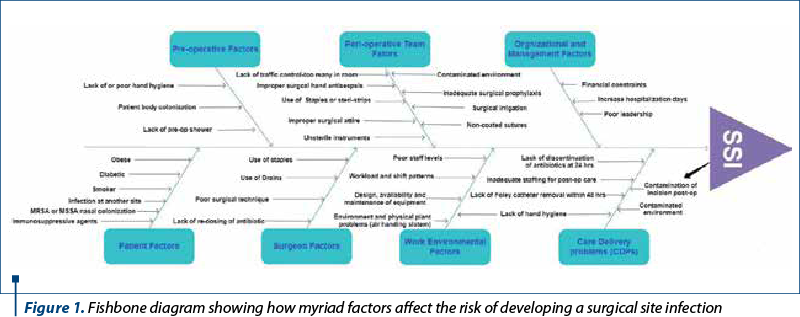
Multiple microbial factors have a direct influence on the establishment of SSI, such as bacterial inoculum, virulence and the consequence of the microenvironment. The infection is produced when these microbial factors are conducive and impaired host defenses set the stage for establishing a chain of events. The patient’s endogenous flora, which is present on the skin, mucous membranes or hollow viscera, contaminates the majority of surgical site infections, but occasionally microorganisms are introduced via surgical instruments, environment or contaminated implants (exogenous)(7). Foreign bodies – such as suture, mesh, adhesion barrier, or even staples, all things that are common in gynecologic surgery – increase the odds for causing a SSI. The microorganisms in gynecologic surgery are unique as the site of infection may be from the vaginal/genitourinary tract, the abdomen or the skin. The most frequent infections in abdominal SSI in gynecologic surgery are with aerobic Gram-positive cocci (Staphylococcus aureus, Staphylococcus epidermidis), although incisions made around the perineum and the groin can also be infected with Enterococcus species and Escherichia coli(8).
Patient characteristics impact the risk of SSI development. A significant problem is signaled by the increasing rates of obesity, as there are many ways in which obesity contributes to infection (limited surgical visualization, poor nutritional status, prolonged time of surgery, lower oxygenation of tissues and reduced antibiotic penetration) and has consistently been associated with increased rates of SSI. Tabacco use has an important effect on tissue ischemia and delayed wound healing, leading to increased rates of SSI. Prolonged operative time has a direct influence on SSI rates, possibly due to temperature regulation, inflammation and anesthesia management. Hyperglycemia in diabetics is a well-known risk factor for several surgical complications, including SSIs(9).
About two-thirds of SSIs are superficial, and the rest are deep and in organs or in intraperitoneal or retroperitoneal spaces. Incisional cellulitis and vaginal cuff cellulitis represent the most common superficial SSI. Deep tissue abscess represents the most common deep tissue infection, whilst tubo-ovarian abscesses and pelvic abscesses constitute the most common space and organ infections. The most serious form of SSI is necrotizing fasciitis, a life-threatening complication if not treated quickly and appropriately(8).
An infection affecting the superficial tissues located at the vaginal surgical margin subsequent to vaginal hysterectomy is represented by vaginal cuff cellulitis. The patient’s symptomatology after hospital discharge consists of moderate but increasing lower abdominal pain with purulent yellow vaginal discharge. Physical examination reveals the vaginal surgical margin to have an out of proportion sensibility with hyperemia and oedema. The adnexa and parametria are left unharmed. The treatment is represented by oral antibiotic therapy with a single broad-spectrum agent with close follow-up in order to assure treatment efficacy(8).
Pelvic cellulitis is typically associated with fever, abdominal pain or the sensation of pelvic fullness. The patient usually presents 5 to 10 days after surgery. The associated symptoms may include anorexia, without gastrointestinal or urinary implications. Physical examination reveals regional sensibility to palpation associated with edema in the absence of masses or peritoneal signs. Ultrasonography will show no masses. Hospitalization is indicated and the treatment should imply intravenous broad-spectrum antibiotic regimen until fever would be resolved for 24-48 hours, and the patient may be discharged on an oral antibiotic regimen with coverage for Gram-positive, Gram-negative and anaerobic bacteria(8).
Pelvic abscesses represent a rare but serious complication of pelvic surgery, occurring when pelvic cellulitis or pelvic hematoma spread into the parametrial soft tissue. Pelvic abscess symptomatology is similar to pelvic cellulitis, with an additional palpable mass corresponding to the collection of infected fluid. The paraclinic investigations pointing out the mass could be ultrasonography, computed tomography (CT) or magnetic resonance imaging (MRI). The patient should be hospitalized, and the treatment with broad-spectrum antibiotics intravenously should be started as soon as possible, until the patient is afebrile for 48-72 hours. Drainage can be accomplished using ultrasound or CT guidance, laparoscopy or laparotomy(8).
Antimicrobial prophylaxis recommendations for gynecologic procedures are outlined in Table 2. For most antibiotics, including cefazolin, prophylaxis should be administered within one hour before skin incision. There are three circumstances in which additional antimicrobial prophylaxis dosages or increased doses may be needed: obesity, lengthy procedure, excessive blood loss(10).
According to ACOG Practice Bulletin No. 195, the most important recommendations for the prevention of infection after gynecologic procedures are(11):
-
Implement perioperative glycemic control and use blood glucose target levels of less than 200 mg/dL in patients with and without diabetes.
-
Preoperative surgical site skin preparation should be performed with an alcohol-based agent unless contraindicated. An appropriate choice remains chlorhexidine-alcohol.
-
Single-dose antimicrobial prophylaxis should be given to patients undergoing vaginal, abdominal, laparoscopic or robotic hysterectomy, including supracervical hysterectomy.
-
Before IUD insertion, routine antibiotic prophylaxis is not recommended.
-
Women undergoing uterine evacuation for induced abortion should be administered antimicrobial prophylaxis.
A reduction in readmission rates and morbidity for surgery can be managed using perioperative „bundles”. The use of checklists greatly facilitates the implementation of SSI bundles. Both Strong for Surgery campaign and the World Health Organization offer surgical safety checklists. Enforcing checklists can help to manage antibiotic re-dosing administration in the appropriate timeframe, as well as monitoring compliance. It has been demonstrated that the implementation of gynecologic perioperative bundles reduces the surgical site infection relative risk with 77.6% in ovarian cancer with bowel resection, with 79.3% in ovarian cancer without bowel resection, and with 100% in uterine cancer(12).
Conclusions
Even though the goal of every surgeon is to prevent wound infections, they still have a tendency to appear. If a SSI develops, the treatment often involves opening, evacuating suppuration, and cleansing the wound. The inspection of more profound tissues might be necessary for integrity evaluation and discovering a deep space infection or source. Dressing changes allow the tissues to granulate, favoring wound healing over several weeks. The infected wound might suffer a delayed closure in case of wound dehiscence, as well as relapse of the infection.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
-
WHO. Global guidelines for the prevention of surgical site infection. 2018, 2nd ed. https://apps.who.int/iris/bitstream/handle/10665/277399/9789241550475-eng.pdf?ua=1
-
Sattar F, Sattar Z, Zaman M, Akbar S. Frequency of post-operative surgical site infections in a tertiary care hospital in Abbottabad, Pakistan. Cureus. 2019; 11(3):e4243.
-
WHO. Protocol for surgical site infection surveillance with a focus on settings with limited resources. 2018. Available at: https://www.who.int/infection-prevention/tools/surgical/SSI-surveillance-protocol.pdf
-
Andiman SE, Xu X, Boyce JM, et al. Decreased surgical site infection rate in hysterectomy: Effect of a gynecology-specific bundle. Obstet Gynecol. 2018; 131(6):991-9.
-
National Healthcare Safety Network (NHSN). Patient safety component manual. Surgical site infection (SSI) event. 2020; 9. Available at: https://www.cdc.gov/nhsn/pdfs/pscmanual/pcsmanual_current.pdf
-
Edmiston CE Jr, Spencer M. Going forward: preventing surgical site infections in 2015. AORN J. 2014;100:616-9.
-
Singhal H. Wound infection treatment & management. 2019. Available at: https://emedicine.medscape.com/article/188988-treatment
-
Lachiewicz MP, Moulton LJ, Jaiyeoba O. Pelvic surgical site infections in gynecologic surgery. Infect Dis Obstet Gynecol. 2015;614950.
-
Steiner HL, Strand EA. Surgical site infection in gynecologic surgery: pathophysiology and prevention. AJOG. 2017;217(2):121-8.
-
Pereira N, Hutchinson AP, Lekovich JP, Hobeika E, Elias RT. Antibiotic prophylaxis for gynecologic procedures prior to and during the utilization of assisted reproductive technologies: a systematicreview. J Pathog. 2016;2016:4698314.
-
ACOG Practice Bulletin No. 195: Prevention of infection after gynecologic procedures. Obstet Gynecol. 2018;131(6):e172‐e189.
-
Johnson MP, Kim SJ, Langstraat CL, et al. Using bundled interventions to reduce surgical site infection after major gynecologic cancer surgery. Obstet Gynecol. 2016;127(6):1135‐44.





