Krukenberg tumor, also known as carcinoma mucocellulare, refers to a rare metastatic signet ring cell tumor of the ovary, the most common primary site being classically the gastrointestinal tract (the gastric carcinoma is responsible for 50% of Krukenberg tumors). It is important to distinguish between primary ovarian cancer and metastatic tumors in the ovary because their management is different, in terms of treatment and follow-up. We report the perioperative management of a 40-year-old female patient with bilateral Krukenberg tumors.
Managementul perioperator al unui pacient cu tumoră Krukenberg - studiu de caz
Perioperative management of a patient with Krukenberg tumor - a case report
First published: 05 octombrie 2017
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/Gine.17.3.2017.1086
Abstract
Rezumat
Tumora Krukenberg, cunoscută şi sub denumirea de carcinom mucocelular, se referă la o tumoră metastatică inelară rară a ovarului, localizarea primară cea mai frecventă fiind tractul gastrointestinal (carcinomul gastric este responsabil pentru 50% din tumorile Krukenberg). Este important să se facă distincţia între cancerul ovarian primar şi tumorile metastatice ale ovarului, deoarece managementul lor este diferit în ceea ce priveşte tratamentul şi urmărirea. Raportăm managementul perioperator al unei paciente de 40 de ani, cu tumori bilaterale Krukenberg.
Introduction
Ovarian tumors comprise a heterogeneous group of lesions, displaying distinct tumor pathology and oncogenic potential and being subclassified into several categories based on two criteria: the degree of epithelial proliferation and invasion and the histotype of the epithelium composing the tumors(1). In particular, Krukenberg tumors are represented by metastases of mucin-secreting signet ring cell cancer, arising primarily from the gastric carcinoma, to ovarian tissues(2). Krukenberg tumors accounts for 1% to 2% of all ovarian neoplasms, with an estimated incidence of 0.16 per 100000 per year(3), and it can be seen in all age groups, with an average age of 45 years(4).
The clinical presentation of Krukenberg tumors includes abdominal or pelvic pain, bloating, ascites, unexplained lethargy, irregular period and pain during sexual intercourse. Krukenberg tumors can occasionally provoke a reaction of the ovarian stroma which leads to hormone production, that results in vaginal bleeding, a change in menstrual habits, hirsutism, or occasionally virilization as a main symptom(5,6).
Regarding the paraclinical diagnostic, most imaging features are non-specific, consisting of predominantly solid components or a mixture of cystic and solid areas; typically, those tumors are described sonographically as bilateral ovarian masses, with an irregular hyperechoic solid pattern, with clear well defined margins and moth-eaten cyst formation(7).
Krukenberg tumor is considered as a late-stage disease with poor prognosis and may account for 30-40% of metastatic cancers to the ovaries(8). Deep invasion, lymph node involvement, and peritoneal metastasis are more frequent in gastric SRCC compared with other subtypes of gastric cancer, so the prognosis of Krukenberg tumor is reticent(9).
Case report
We report the case of a 40-year-old female patient, without a significant pathological personal history, who has been admitted two months ago in the Department of Gynecology of a regional hospital, accusing pelvic pain and dysfunctional menstrual cycles. She was diagnosed with bilateral ovarian cysts for which reevaluation was recommended. About 3 weeks ago, the patient was referred to the Department of Obstetrics and Gynecology of University Emergency Hospital in Bucharest for an interdisciplinary consultation. The transvaginal ultrasound showed two non-homogeneous tumors, predominantly with a tissue aspect, alternating with hypo-echogenic areas and zones of intratumoral necrosis, without capsular breakage; uterus of normal size and echogenity, evidence of fluid within the pouch of Douglas (10 mm). CA125 tumor markers were recommended. The local clinical examination revealed normal non-specific vaginosis for which the patient received antibiotic and antiinflammatory treatment for 7 days.
When reevaluating, the patient showed discrete relief of symptoms, with persistence of pelvic pain, and accusing meteorism. Laboratory findings showed normal CA125 (12 U/ml) and normal cervico-vaginal cytology. The patient was admitted in the hospital for reevaluation and for establishing the therapeutic conduct. New laboratory findings showed elevated values of tumoral markers: CA125 (57 U/ml), CEA (10.7 ng/ml) and CA19.9 (0.4 U/ml). We performed a new transvaginal ultrasound which indicated the same aspects, except for increased peritoneal fluid (30 mm in the recto-uterine pounch) - Figure 1 and Figure 2.
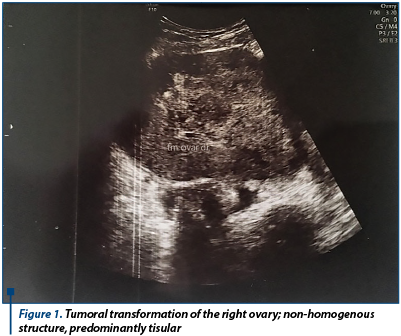
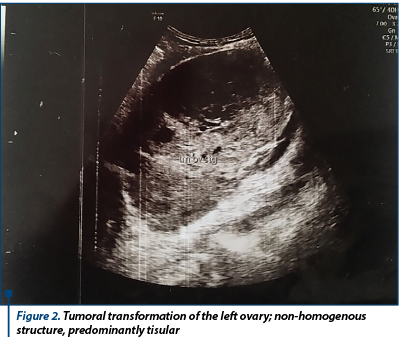
A coagulopathy was also detected, which raised the suspicion of a liver disease and elevated inflammatory markers (Fibrinogen 719 mg/dl) with negative infectious markers. A computed tomography of the thorax, abdomen and pelvis was performed; it detected bilateral ovarian tumors, measuring 130/82 mm on the left side and 130/79 mm on the right side, with a mixed solid-cystic component, predominantly solid, non-homogeneous, iodophilic, which was difficult to differentiate between primitive ovarian tumors and ovarian metastases; the tumors also compressed the rectosigmoid junction, but without overly occlusive appearance; also, multiple hypo-dense and hypo-attenuating nodular lesions, having a maximum size of 32/27 mm, suggestive for the presence of liver metastases were detected - Figure 3 and Figure 4.
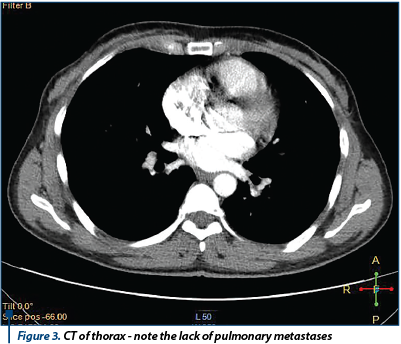
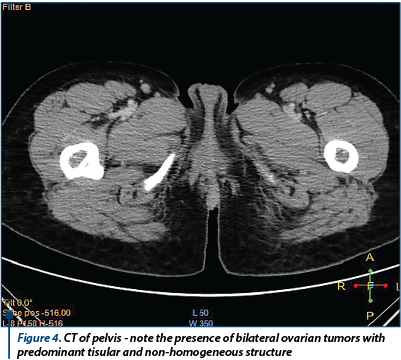
The general condition of the patient deteriorated, with the occurrence of vomiting and pain in the right hypochondria and the epigastrium. General surgery consultation was requested to exclude a sub-occlusive syndrome, followed by upper endoscopy which showed a normal aspect, with the exception of enlarged folds in the vertical portion of the stomach, but which distended fully under insufflation. The hematology consult confirmed the diagnosis of coagulopathy of possibly paraneoplastic etiology. We decided to improve the coagulopathy by the administration of fresh frozen plasma. Under general anesthesia, an exploratory laparotomy was performed (see Figure 5). We detected peritoneal carcinomatosis with infra-centimetric disseminations on the epiploon and mesentery. The ovaries were transformed tumoraly, without capsular breakage, measuring 12/10 cm (the left one) and 10/10 cm (the right one). We also observed free peritoneal fluid in a small amount and multiple liver metastases with various sizes (1-3 cm).
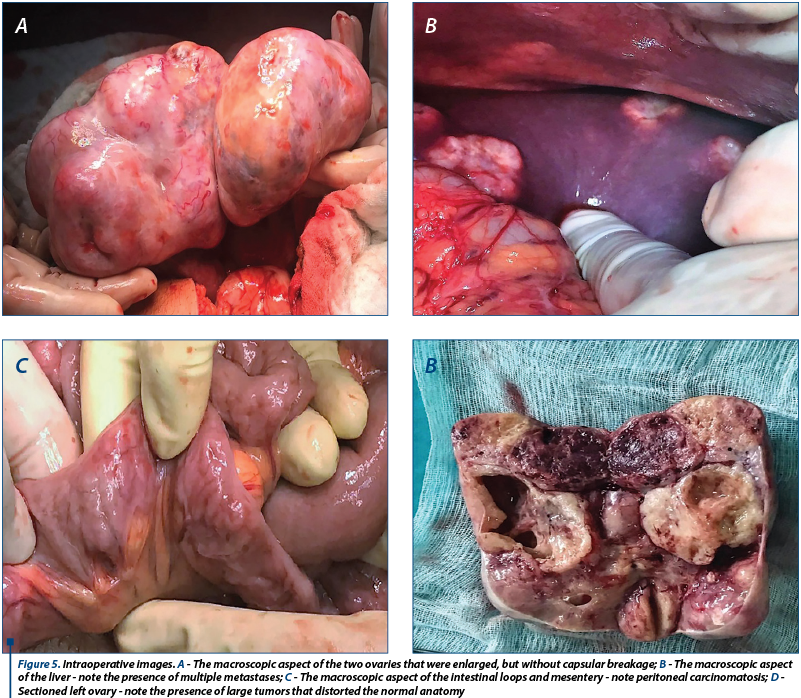
We decided and practiced tumor cytoreduction through total hysterectomy with bilateral oophorectomy, with the piece being sent to histopathological examination (histopathological extemporaneous examination showed undifferentiated ovarian carcinoma with Mullerian cells); tactical omentectomy and biopsy of all secondary lesions were also performed. The postoperative evolution was favorable with the improvement of genital symptomatology; the patient was discharged after 5 days and she was guided to the Oncology Department to follow the specialized treatment after receiving the final histopathological result.
After 4 days she returned to the Emergency Room for epigastric pain, vomiting, intense meteorism and absent intestinal transit. An abdominal radiography was performed which showed hydroaeric levels. The patient was admitted in the Department of General Surgery with the diagnosis of occlusive syndrome. A surgical reintervention in a multidisciplinary team was performed. Intraoperatively, we found an early adherence syndrome.
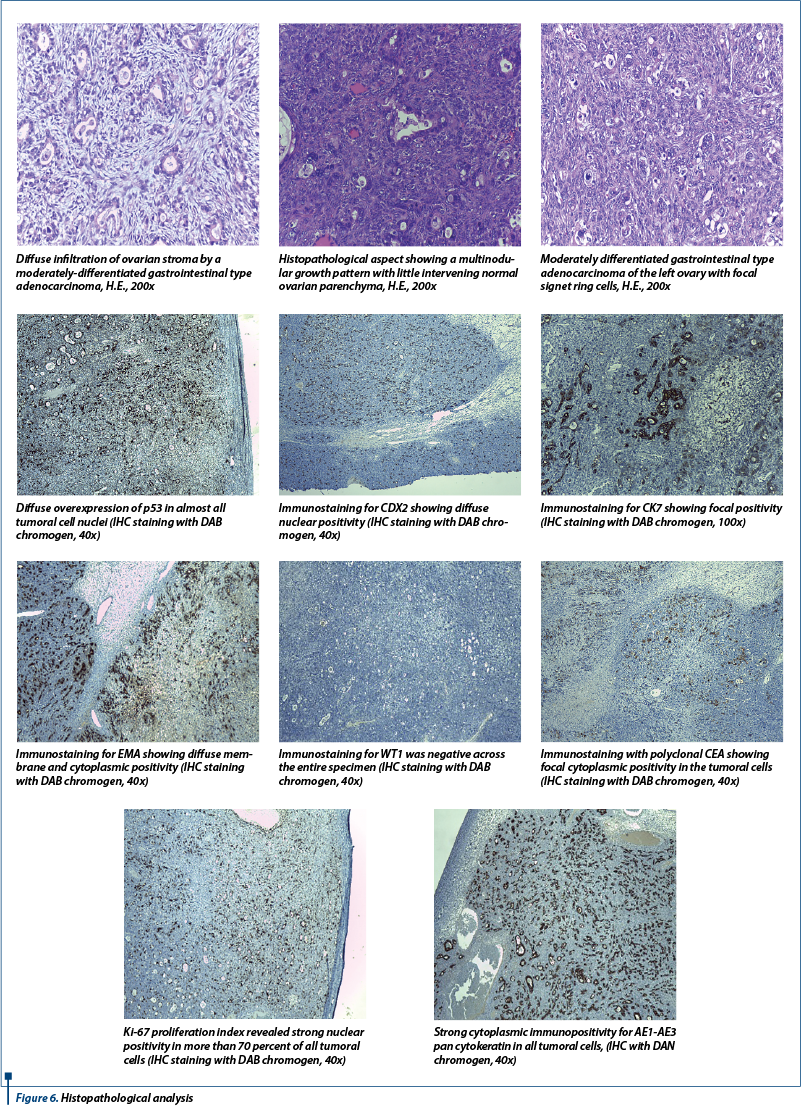
After an extensive histopathological analysis which included multiple immunohistochemistry tests, the diagnosis of Krukenberg tumors was established (Figure 6).
The postoperative evolution was favorable, with improvement of digestive symptomatology; the patient will perform other specialized investigation (echo-endoscopy) and she was guided to the Oncology Department for specific postoperative treatment.
Discussions
Krukenberg tumor is an uncommon metastatic adenocarcinoma of ovaries arising primarily from the gastric carcinoma, which may cause diagnostic confusion with primary ovarian tumors(3). The first mention of the existence of this kind of tumor was in 1896, when Krukenberg presented five cases of peculiar ovarian tumors having appearance of malignant cells as new type of primary ovarian sarcomas, which he named “fibrosarcoma ovarii mucocellulare (carcinomatodes)”(10). Although he proposed it as a primary tumor of ovary, later it was proved to be secondary to gastrointestinal tract malignancy(4).
About 80% of Krukenberg tumors are bilateral and range in size from 5 to 20 cm. Ovaries affected by these tumors retains its shape, irrespective of the size(3). Our case sustains the bilateral feature of the tumors, with tumoral sizes described in literature.
Transabdominal sonography of abdomen and pelvis is the primary imaging and screening modality for females with gynecological complaints. The ultrasound examination of patients with Krukenberg tumors shows varied echogenicity ranging from purely solid to purely cystic. In contrast with the primary ovarian tumors in which criteria used to describe the ovarian malignancy (irregular solid tumor, ascites, at least 4 papillary structures, multi-loculated solid tumor with the largest diameter over 100 mm and the presence of increased Doppler flow), most frequently, Krukenberg tumors will be homogenously hyperechoic solid masses with few cysts within. There will be large lead vessel penetrating the mass from the periphery and nourishing the tumour by branching in tree pattern, known as lead vessel sign, with high speed and low resistance on spectral Doppler(3,11,12).
During the histopathological analysis, these tumors are characterized by the presence of signet ring cells and pseudo-sarcoma proliferation of ovarian stroma(13). Immunohistochemical tests have a large impact on the diagnostic of ovarian carcinomas, by providing useful assessment criteria for a better reproducibility of cell type diagnosis(15). For a good differentiation of the histological subtype and for assessing tumor aggressiveness, it is necessary to conduct immunohistochemical tests, which commonly target the expression of proliferation markers and aggression CK7, WT1, p53 and ki67(16-18). We conducted an extensive histopathological examination and also performed multiple immunohistochemistry tests in order to establish the final diagnosis of Krukenberg tumor.
The presence of Krukenberg tumors at the time of imaging poses extremely poor prognosis for patients, with a survival rate ranging from 12% to 23.4%(14).
Conclusion
The management of a patient with a Krukenberg tumor requires an interdisciplinary approach, which includes well trained specialists in imagistics, gynecology and general surgery. Due to the fact that imagistic methods and intraoperative aspect are nonspecific, an extensive histopathological analysis with immunohistochemistry tests, performed by a specialist in Pathology, is mandatory in order to establish the diagnosis.
Bibliografie
2. Kiyokawa T, Young RH, Scully RE. Krukenberg tumors of the ovary: a clinicopathologic analysis of 120 cases with emphasis on their variable pathologic manifestations. Am J Surg Pathol.2006; 30:277–99.
3. Hiremath R, Padala KP, Gouda MG, Pailoor A. Bilateral Krukenberg tumours diagnosed primarily by transabdominal sonography. A case report. J Clin Diagn Res, 2015; 9(12): TD01–03.
4. Al-Agha OM, Nicastri AD. An in-depth look at Krukenberg tumor: An overview. Archiv Path Lab Med. 2006; 130(11):1725–30.
5. Kiyokawa T, Young RH, Scully RE. Krukenberg tumors of the ovary: a clinicopathologic analysis of 120 cases with emphasis on their variable pathologic manifestations. Am J Surg Pathol. 2006; 30:277–99.
6. Papakonstantinou E, Liapis A, Kairi-Vassilatou E, Iavazzo C, Kleanthis CK, Kondi-Pafiti A. Virilizing ovarian Krukenberg tumor in a 27-year-old pregnant woman: a case report and literature review. Eur J Gynaecol Oncol. 2011; 32:331–3.
7. Shimizu H, Yamasaki M, Ohama K, Nozaki T, Tanaka Y. Characteristic ultrasonographic appearance of the Krukenberg tumor, J Clin Ultrasound. 1990; 18(9):697-703.
8. Lu W, Yuan L, Liu X, Guo SW. Identification of prognostic factors for Krukenberg tumor. GMIT. 2013; 2:52–6.
9. Li C, Kim S, Lai JF, Hyung WJ, Choi WH, Choi SH, Noh SH. Advanced gastric carcinoma with signet ring cell histology. Oncology. 2007; 72:64–8.
10. Das S, Sahu D, Wani M, Reddy PK. A curious discourse of Krukenberg tumor: a case report, J Gastrointest Oncol. 2014; 5(6): E117–E120.
11. Brătilă E, Coroleucă CB, Coroleucă CA, Comandaşu DE, Mehedinţu C, Bohîlţea R, Cîrstoiu MM, Mitran M, Berceanu C. The role of Doppler examination in evaluating the ovarian pathology. Ginecologia.ro, 2016; 4(14):46-54.
12. Spinelli C, Liloia C, Piscioneri C, Ugolini C, Strâmbi S. An unusual evolution of Krukenberg tumour. A case report. J Clin Diagn Res. 2016; 10(10): PD07–11.
13. Irving JA, Lerwill MF, Young RH. Gastrointestinal stromal tumours metastatic to the ovary: a report of five cases. Am J Surg Pathol. 2005; 29(7):920–26.
14. Testa CA, Licameli A, Legge AD, Mascilini F, Pelagalli M, Scambia G. Colour Doppler sonographic features of Krukenberg tumour in pregnancy. J Ultrasound Med. 2009; 28:695–98.
15. Stănescu AD, Pleş L, Edu A, Olaru GO, Comănescu A, Potecă AG, Comănescu MV. Different patterns of heterogeneity in ovarian carcinoma. Rom J Morphol Embryol. 2015; 56(4):1357–63.
16. Brătilă E, Manta AM, Strâmbu VDE, Iorga CIM, Comandaşu D, Mehedinţu C, Vlădăreanu S, Berceanu C, Cîrstoiu MM, Mitran M. Therapeutic conduct in borderline ovarian tumors in women at fertile age. Ginecologia.ro, 2016; 4(11):56-60.
17. Liliac L, Carcangiu ML, Canevari S, Căruntu ID, Ciobanu AD, Danciu M, Amălinei C. The value of PAX8 and WT1 molecules in ovarian cancer diagnosis. Rom J Morphol Embryol, 2013; 54(1):17-27.
18. Mărinaş MC, Mogoş DG, Simionescu CE, Stepan A, Tănase F. The study of p53 and p16 immunoexpression in serous borderline and malignant ovarian tumors. Rom J Morphol Embryol, 2012; 53(4):1021-5.
Articole din ediţiile anterioare
Clear cell carcinoma arising from endometriotic ovarian cyst
Carcinomul cu celule clare este un subtip rar şi agresiv de cancer ovarian. Este adesea asociat cu endometrioza, o afecţiune cronică ce este caract...
Utilitatea imunohistochimiei în diagnosticul carcinomului ovarian
Cancerul ovarian este o problemă de sănătate publică ce afectează femeile de vârstă reproductivă, fiind o cauză majoră a morbidității și mortalit...