Objective. The results of the prenatal diagnosis of chromosomal anomalies performed during a five-year period are presented and analyzed, in order to determine the frequency of fetal aneuploidies. Methodology. We used quantitative fluorescence polymerase chain reaction (QF-PCR) for testing the fetal DNA, and we calculated the percentage of each chromosomal anomaly. Results. The most frequent anomalies found were trisomies 21 and 18. We discuss the problems that might cause discrepancies between the results obtained with QF-PCR and other techniques. Conclusions. This study underlines the utility of the QF-PCR analysis for the prenatal diagnosis of aneuploidies and the importance of karyotype that remains the best solution for the final decision regarding the pregnancy, especially when there were detected echographic anomalies.
The prenatal diagnosis of chromosomal anomalies by quantitative fluorescence polymerase chain reaction in Romania – a five-year report from a new institute
Diagnosticul prenatal al anomaliilor cromozomiale prin reacţia cantitativă de polimerizare în lanţ cu fluorescenţă în România – un raport pe cinci ani al unui nou institut
First published: 30 martie 2023
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/Gine.39.1.2023.7782
Abstract
Rezumat
Obiective. Rezultatele diagnosticului prenatal al anomaliilor cromozomiale efectuat pe o perioadă de cinci ani sunt prezentate şi analizate pentru a determina frecvenţa aneuploidiilor fetale. Metodologie. Am folosit tehnica QF-PCR pentru testarea ADN-ului fetal şi am calculat procentajul fiecărei anomalii cromozomiale. Rezultate. Cele mai frecvente anomalii constatate au fost trisomiile 21 şi 18. Sunt discutate problemele care ar putea provoca discrepanţe între rezultatele obţinute cu QF-PCR şi alte tehnici. Concluzii. Acest studiu subliniază utilitatea analizei QF-PCR pentru diagnosticul prenatal al aneuploidiilor şi importanţa cariotipului, care rămâne cea mai bună soluţie pentru decizia finală cu privire la sarcină, mai ales atunci când au fost detectate anomalii ecografice.
Introduction
The prenatal diagnosis of chromosomal anomalies is of high importance in cases of pregnancies with increased risk, such as the mother’s age over 35 years old, abnormal results of biochemical screening tests, fetal malformations, deficitary development, other pregnancies or children with chromosomal anomalies or genetic mutations, dead fetuses, and other pathological conditions. In the IMOGEN Institute, within the Cluj County Emergency Clinical Hospital, the prenatal diagnosis of aneuploidies and other chromosomal anomalies was developed on the basis of the previous experience obtained in the Cytogenetics Laboratory which was established in the First Obstetrics and Gynecology Clinic from Cluj-Napoca. The prenatal karyotype has a relative long working time, and a rapid test is necessary in order to confirm the biochemical tests’ results, such as the Double or Triple Test, and to prevent the anxiety of the patients. Quantitative fluorescence polymerase chain reaction (QF-PCR)(1,2) method for the most common aneuploidies was introduced as a diagnosis test in our laboratory. We present and analyze the results obtained with this method.
Materials and method
The amniotic fluid was the most frequently used for the DNA extraction. A small proportion of the samples consisted of chorion villi, or fragments from aborted fetuses. The patients signed an informed consent for collection and usage of their biological samples for diagnosis and research. The samples of amniotic fluid were collected by amniocentesis during the week 16-20 of pregnancy. The DNA was extracted with the PureLink Genomic DNA mini kit from Invitrogen and quantified at 230, 260 and 280 nm with a spectrophotometer (EPOCH, manufactured by BIOTEK, or Qubit 3.0 Fluorometer, manufactured by Life Technologies Corporation). The DNA concentration was adjusted in order to use around 3.5-5 ng of DNA in the QF-PCR. The DNA samples were analyzed with the Devyser Compact and Devyser Resolution v3.0 kits from Devyser Inc. The amplification of the STR markers by PCR was performed according to the manufacturer instructions with an Applied Biosystems Veriti thermocycler with 96 wells, and the samples were analyzed with an ABI Prism 310 DNA genetic analyzer manufactured by Applied Biosystems and Perkin-Elmer. The results were processed and analyzed with the GeneMapper v.4.0 (Applied Biosystems) (Figures 1-3), and the interpretation of the results was performed according to the manufacturer instructions(3) and the international professional guidelines(4-6).
A total of 1086 pregnant women were analyzed by QF-PCR in a five-year period, between 2015 and 2019.
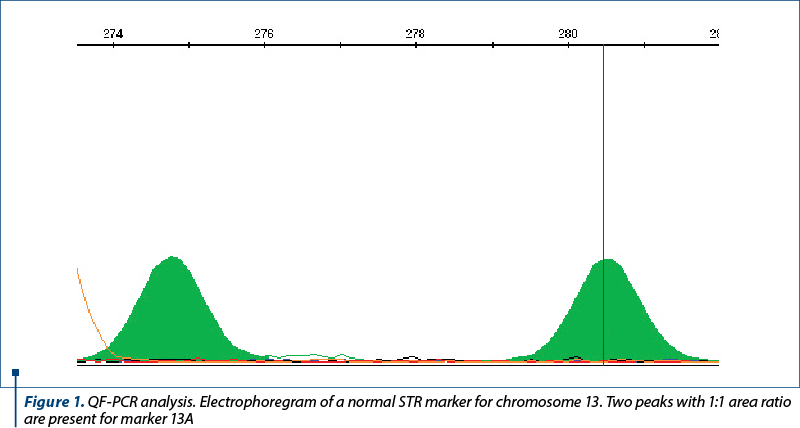
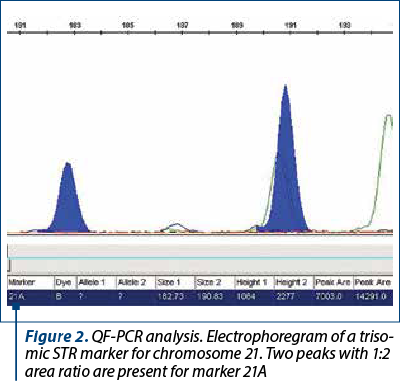
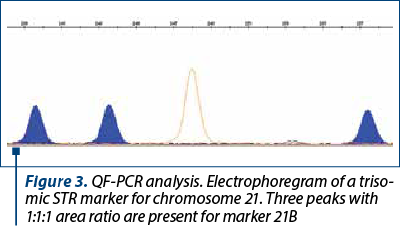
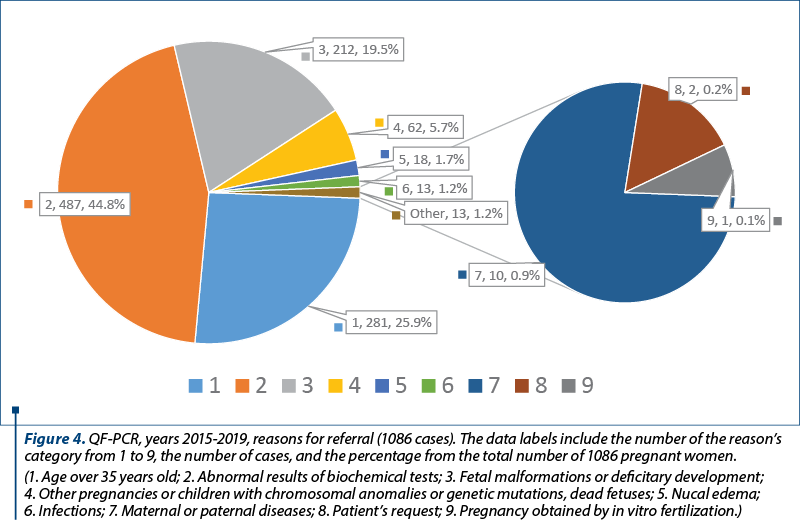
The reasons for referral of pregnant women to the prenatal diagnosis for aneuploidies by QF-PCR were taken from the register (Figure 4). When there was more than one reason for referral, the most important was used for the classification. For example, the fetal malformations were considered the main reason, compared with the age or abnormal results of biochemical tests. Generally, the first reason mentioned in the register was considered the most relevant. This study has been approved by the Ethics Committee of the hospital.
Results
The results of the QF-PCR analysis are presented in Figure 5.
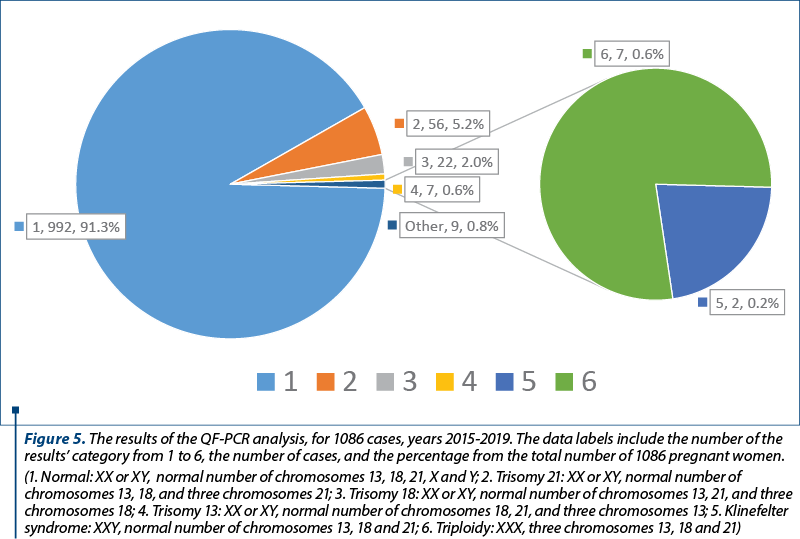
In order to correlate these data with the results of other investigations performed for the patients, we mention that, during a five-year period, between 2015 and 2019, 10,971 pregnant women were investigated by double or triple screening tests. The Double Marker Test consists in the concentration measurement of two markers, namely Free Beta human Chorionic Gonadotrophin (hCG) and Pregnancy Associated Plasma Protein A (PAPP-A). The triple screening test consists in the determination of the serum levels of alpha-fetoprotein, estriol and beta-hCG. There were found 417 abnormal results (3.8%). The investigations continued in our service for 270 out of these 417 pregnancies, and nine cases (3.3%) were confirmed with a chromosomal anomaly by QF-PCR and/or karyotype.
The pregnant women were also investigated by echography, and in 399 cases anomalies were found. These patients were investigated by QF-PCR and/or karyotype, and 70 (17.5%) chromosomal anomalies were detected. Out of these 399 cases, 230 were analyzed by QF-PCR. They were classified in the categories of reasons for referral number 3 and 5 (fetal malformations, deficitary development, nucal edema) – Figure 4.
Discussion
In almost all cases analyzed, the results were consistent with the karyotype results (unpublished data), taking into consideration the limits of QF-PCR, in which only the aneuploidies of chromosomes 13, 18, 21, X and Y are analysed; therefore it cannot detect aneuploidies of other chromosomes and structural anomalies.
There were few discrepancies between the results obtained by QF-PCR and karyotype.
One case with fetal malformations (fetal hydrops, cystic hygroma, amniotic bride) was referred to QF-PCR and karyotype. At the QF-PCR analysis of the chorionic villi, the fetus was female, with most of the sex markers being uninformative, but one was normal (the T1 marker, which represents the proportion between the chromosomes 7 and X) and, consequently, did not meet all the criteria for a chromosomal anomaly. The culture for karyotype failed, the patient did not return for amniocentesis and gave birth to a girl with Turner syndrome (45, X). Another case was referred to QF-PCR and karyotype for suspicion of Down syndrome. The analysis of the chorionic villi sample by QF-PCR was normal, but the prenatal karyotype of the amniotic cells indicated trisomy 21. This discrepancy was probably caused by a limited mosaicism of the sample analyzed by QF-PCR. The third case was analyzed with QF-PCR, being a normal female (46, XX), but the sample of amniotic fluid was contaminated with a high amount of blood. The karyotype result was 47, XXY, corresponding to the Klinefelter syndrome.
Conclusions
This study underlines the utility of the QF-PCR analysis for the prenatal diagnosis of aneuploidies, and reveals once again the relative imprecision of the analysis of the chorionic villi compared with the amniotic fluid and the false results in case of significant contamination of the amniotic fluid with maternal blood. We consider that all the QF-PCR results obtained on chorionic villi, which are, however, more difficult to interpret compared with other categories of prenatal samples, should be taken into consideration with caution, and in all these cases the amniocentesis should be performed, in order to prevent any errors of diagnosis. The results obtained on samples contaminated with maternal blood should also be interpreted with caution, and the level of contamination should be determined. In our opinion, the karyotype remains the best solution for the final decision about the pregnancy, especially when there were detected echografic anomalies.
Acknowledgements. This work was supported by the European Regional Development Fund, Sectoral Operational Program “Increasing Economic Competitiveness” Priority Axis 2 – “Research, Technological Development and Innovation for Competitiveness” (European Union, Romanian Government, Sectoral Instruments 2007-2013) and by the National Health Program “Mother and Child. Prenatal and Postnatal Diagnosis”.
We thank to biologist Iulia Mândruţiu, technicians Maria Szalai, and Simona Petric from the IMOGEN Institute for performing the laboratory work (DNA extraction, PCR and electrophoresis).
The topic is covered by reports concerning the utility of quantitative fluorescence polymerase chain reaction (QF-PCR) technique and the limits of the method.
There are available limited data about the frequency of fetal chromosomal anomalies in Romania.
This study provides data about the incidence of the fetal aneuploidies in the Romanian population.
Data Availability Statement: research data are not shared.
Conflict of interest: none declared
Financial support: none declared
This work is permanently accessible online free of charge and published under the CC-BY.

Bibliografie
-
Mann K, Ogilvie CM. QF-PCR: application, overview and review of the literature. Prenat Diag. 2012;32(4):309-14.
-
Mann K. An introduction to QF-PCR. Available at: https://devyser.com/knowledge-hub/introduction-to-qf-pcr
-
Devyser Compact v.3 manual. Available at: https://devyser.datadwell.com/link/1407-devyser-compact-v3-en-ruo-v2-2013.pdf/play?player=1.
-
Professional Guidelines for Clinical Cytogenetics and Clinical Molecular Genetics, QF-PCR for the Diagnosis of Aneuploidy, Best Practice Guidelines (2012) v3.01. Available at: https://www.researchgate.net/publication/296269288_ACCCMGS_best_practice_guidelines_for_QF-PCR-based_diagnosis_of_aneuploidy
-
Mann K. Detecting Mosaicism with QF-PCR. Available at: https://devyser.com/knowledge-hub/detecting-mosaicism-with-qf-pcr
-
Mann K. Detection of Maternal Cell Contamination by QF-PCR. Available at: https://devyser.com/knowledge-hub/qf-pcr-and-maternal-cell-contamination
Articole din ediţiile anterioare
Prenatal diagnosis of micrognathia
Early detection of micrognathia during prenatal care is crucial to mitigate the associated risks and prevent unforeseen emergencies requiring inv...
Ultrasound-based differential diagnosis of fetal abdominal wall defects in early pregnancy
Fetal abdominal wall defects (AWDs) encompass a wide range of congenital anomalies that involve the incomplete closure or disruption of the abdomin...