Rhesus alloimmunization represents a cause for perinatal morbidity and mortality. In fetal severe anemia due to red blood cell alloimmunization, intrauterine blood transfusion and preterm delivery may be considered as a treatment option. Anti-D should be given within 72 hours after birth to reduce the risk of RhD complications in rhesus-negative women who have given birth to a rhesus-positive child.
Incompatibilitatea RH-ului şi rezultatul sarcinii în cazul femeilor cu RH negativ
Rh incompatibility and the pregnancy outcome of Rh negative women
First published: 21 iunie 2019
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/ObsGin.67.2.2019.2419
Abstract
Rezumat
Alloimunizarea Rh reprezintă o cauză a morbidităţii şi mortalităţii perinatale. În anemia severă fetală datorată hemolizării eritrocitelor, conduita curativă constă în transfuzia de sânge intrauterină şi inducerea naşterii înainte de termen. Anti-D trebuie administrat în decurs de 72 de ore după naştere pentru a reduce riscul de complicaţii la femeile rhesus-negative care au dat naştere unui copil rhesus-pozitiv.
Introduction
Red blood cell (RBC) alloimmunization and hemolytic disease of the fetus and newborn (HDFN) remain significant problems in all countries(1).
Feto-maternal red cell alloimmunization is defined by the presence in a pregnant woman of alloantibodies directed against blood group antigens present on the red blood cells of the fetus and inherited from the father(2).
Anti-D is the most common antibody responsible of feto-maternal red blood cell alloimmunization. Once sensitized, future pregnancies may be at risk for hemolytic disease of the fetus and newborn(1).
This review describes Rh incompatibility and the preventative therapies during pregnancy for immunization to RhD.
Intrauterine blood transfusion (IUT) in the management of pregnancy complicated by rhesus sensitization is briefly discussed.
Rh blood group system and non-RhD antigens
Rh antigens are lipoprotein molecules, which are sparsely located at the erythrocyte surface. About 50 of them can be identified, which indicates the specific complexity of the Rh antigen(3).
The genetic conditions in Kell’s system are as complicated as in Rhesus system. However, anti-K alloimmunization rarely occurs due to the very limited frequency of only 9% of factor K(4). D antigen is the most immunogenic; it causes the formation of antibodies 50 times more often than the C and E antigens(3). D factor is so strong that, after transfusion of D-positive blood to D-negative receptors, 80% of the case is associated with immunization(4).
Approximately 0.2-1% of routine RhD blood typings result in a “serological weak D phenotype”. Most serological weak D phenotypes in Caucasians women express molecularly defined weak D types 1, 2 or 3 and can be managed safely as RhD-positive, eliminating unnecessary injections of Rh immune globulin and conserving limited supplies of RhD-negative RBCs(5).
DD and Dd phenotypes are of interest to partners of immunized Rh negative women. So, babies are born Rh positive with the potential for severe anemia when the father is homozygous (DD), while as heterozygous (Dd) there is a chance that 50% of children will be Rh negative and healthy.
The incidence of Rh disease in a population depends on the predominance of rhesus negative. About (15%) of the white population has an RhD negative blood type(6).
Rh immunization is usually caused by a prior Rh-positive fetal maternal transplacental hemorrhage, which occurs in at least 75% of pregnancies(7).
Newborn erythroblastoses due to Duffy antibodies are rare and only described in transfused pre-immunized mothers(4).
Kidd antibodies may cause acute and delayed hemolytic reactions as well as hemolytic disease of the fetus and newborn (HDFN)(8).
Pregnancies complicated by red cell alloimmunization. Screening for red blood cell antibodies
Severe fetal anemia in this setting is owing to Rh incompatibility between fetus and mother. The most common causes of fetomaternal hemorrhage associated with red cell antigen alloimmunization are: ectopic pregnancy, spontaneous abortion, elective abortion, fethal death (any trimester); chorionic villus sampling, amniocentesis, felal blood sampling; delivery, trauma, placental abruption, unexplained vaginal bleeding during pregnancy, and external cephalic version. For each, anti-D immune globulin is recommended(9).
Anti-D and anti-K alloantibodies were implicated in HDFN, a very serious medical condition, which leads to fetal anemia, fetal hydrops, asphyxia and perinatal death. Prevention of fetal hydrops by timely detection and treatment may improve long-term outcome(10); this surveillance aims at screening for severe anemia before hydrops fetalis(11). Hydrops related to anemia is rare in fetuses with hemoglobin concentrations greater than 5 g/dL(12).
The prevalence of rhesus alloimmunization has decreased, so that only one to six cases occurs in every 1000 live births(13).
The risk of Rh D alloimmunization after the first birth is 16% if the fetus is ABO compatible with the mother, 2% if he is not compatible (about 20% of the cases), and 2% after termination of pregnancy; the overall risk of immunization is 13.2%(3).
All pregnant women should be blood-typed and investigated for atypical antibodies in early pregnancy, preferably at the initial prenatal visit(14).
Blood typing for Rh-D, Rh-c, Rh-E and Kell systems is clinical important, because it relates to their ability to produce alloantibodies which can cause hemolysis.
Indirect Coombs test (ICT) is extremely useful for detecting and identifying alloantibodies.
A few reports about antibody screening in pregnant women showed that NaCl/Enzyme method should be used together with LISS/Coombs method. Enzyme-enhanced methods often detect low concentrations of anti-Rh antibodies not found by other methods(15).
The early determination of the increasing anti-D titers is needed to select pregnancies at increased risk for HDFN with indication for transfusion therapy. According to a retrospective study, anti-D quantification does not add further information compared to anti-D titre ≥128, in defining a critical level to start monitoring RhD-immunized pregnancies with Doppler ultrasound(16).
It is recommended that all RhD negative women are routinely offered anti-D Ig prophylactically during antenatal care (at 28 weeks), and following any antenatal ‘potential sensitising event’ where fetal maternal haemorrhage (FMH) may have occurred(17).
Anti-D given to rhesus-negative women within 72 hours, who have given birth to a rhesus-positive child, decreased the likelihood of the women developing rhesus antibodies within six months of the birth and in their next pregnancy(18).
Methods for predicting severe anemia and intrauterine blood transfusion (IUT)
A critical stage of the process is that of blood sampling for pretransfusion testing with the potential for misidentification(19).
Weak or partial D carriers are considered rhesus positive as donor in contrast to transfusion recipient, who are managed as rhesus negative(4).
Red cells selected should be group O D-negative (except where maternal anti-c is present, then provide D-positive, c-negative) and K-negative (except where maternal anti-k [Cellano] is present, then provide k-negative), and should be collected less than 72 hours prior to the planned transfusion, irradiated and also leucodepleted(20).
The decisions for treatment with intrauterine blood transfusion were based on increased peak systolic velocity in the middle cerebral artery (MCA-PSV), the most sensitive predictive parameter of fetal anemia(16).
It can be obtained as early as 16 to 18 weeks gestation. A MCA-PSV greater than 1.5 MoM (multiples of the median) is used as a screening test to identify the severely anemic fetus, and can be directly diagnosed by fetal blood sampling. If the hematocrit is below 30%, IUT is performed. The number of required transfusions may vary between one and six. The final IUT procedure is usually not performed after 35 weeks of gestation, and the patient is scheduled for delivery approximately three weeks after, at 37 to 38 weeks(21). The management of the severely affected fetus consists of early delivery, with or without fetal transfusions, depending on the gestation of the fetus(7).
However, the sensitivity and specificity of this technique may be excellent for the first IUT, but may not be as sensitive in guiding timing of the subsequent procedures. After an initial transfusion, the Society for Maternal-Fetal Medicine recommends higher threshold (MoM>1.69) for the diagnosis of fetal anemia requiring a second transfusion. Troia et al. reported that the 1.69 cutoff as suggested by the Society for Maternal-Fetal Medicine would not have identified all the anemic fetuses that received the second and third IUTs(22).
In intravascular transfusion (IVT), the access into the fetal circulation is obtained under ultrasound guidance using a 20-G needle inserted into either the umbilical vein at its placental insertion, or the intrahepatic umbilical vein; a rate of transfusion of 5 to 10 mL/minute with a minimal risk of moving the needle. The most accessible site usually requires an anterior placenta, in which the cord root can be visualized; the posterior placentation makes the technique more difficult(23,24).
Transfusions are repeated every 14 days or less, with the interval based on the rate of decline in the hematocrit from the previous transfusion. Severely affected fetuses may require transfusions as frequently as every 7 days(24,25).
The quantity of blood to transfuse is determined by the formula: EFW (g) x coefficient = volume to transfuse (Table 1).
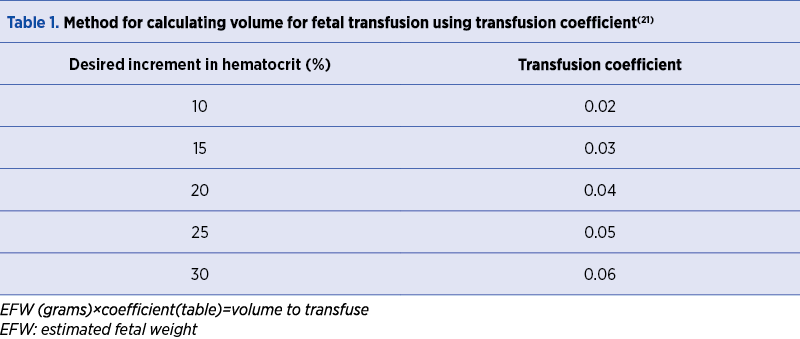
A fetus with hydrops is not able to tolerate severe volume overload, and for this reason the hematocrit should not be increased greater than fourfold. Adjusting transfusion speed to gestational age may improve outcomes in young and/or hydropic fetuses, preventing of volume overload(10).
In general, the final target hematocrit should be approximately 40-50%(21).
Possible complications of IVT include: fetomaternal haemorrhage; fetal loss (1-2%); bradycardia or tachycardia (5%); infection (0.3%); cord haematoma, bleeding from the venipuncture site; cardiac overload; premature rupture of the membranes (0.1%)(24,25).
Other known complications of intrauterine transfusion include splenic rupture and iron overload(24,25). Intrauterine transfusions suppress erythropoiesis and can cause hypoproliferative anemia in the neonatal period(25).
Specific complications due to administration of blood products can be overcome by the selection of blood negative for cytomegalovirus (CMV), hepatitis and HIV(26).
Intraperitoneal transfusion (IPT) is the method of choice below 18 weeks, when direct access to the fetal vasculature is dangerous(24). Approximately 10 mL is transfused for each week of gestation beyond 20 weeks (GA-20)×10(27). This procedure should be avoided if possible in the hydropic fetus(23).
A combined intravascular and intraperitoneal approach may allow longer intervals between procedures(10).
Intracardiac transfusion can be performed in selected cases of fetal terminal anemia with bradycardia, where there is no access to the umbilical cord and IPT is unsuitable(24).
IUT is at high risk and should be used when there are no alternative approaches and there is a clear clinical indication.
Discussion and conclusions
With IUT, the survival of fetuses with alloimmunization is higher than 90%, but 4.8% of children with at least one IUT have neurodevelopmental impairment(11).
It is essential for the blood transfusion laboratory to have information about previous intrauterine transfusion, because it will influence the selection of appropriate blood components(20).
The routine use of middle cerebral artery (MCA) Doppler velocity in the evaluation and monitoring of suspected fetal anemia can lead to less unnecessary invasive procedures.
Women with severe HDFN secondary to maternal RBC alloimmunization were successfully managed with therapeutic plasma exchange (TPE), intravenous immune globulin (IVIG) and IUT(28).
The prevention is extremely important, because once sensitization occurs, rhesus immune globulin is no longer effective(13). The 100 µg dose prevents immunization by up to 4-5mL RhD-positive RBCs, that is, about 99% of all fetomaternal hemorrhages. Despite these measures, RhD immunization still occurs, probably as a result of spontaneous fetomaternal hemorrhages (0.25 ml of fetal blood is the minimum amount required to lead to alloimmunizatin)(14).
The management of rhesus alloimmunization in pregnancy implies close cooperation between obstetricians and a multidisciplinary team of medical specialists.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
2. Bricca P, Guinchard E, Guitton Bliem C. Management of feto-maternal red cell allo-immunizations. Transfus Clin Biol. 2011; 18(2):269-76. doi: 10.1016/j.tracli.2011.01.005.
3. Izetbegovic S. Occurrence of ABO And RhD Incompatibility with Rh Negative Mothers. Mater Sociomed. 2013; (4):255-8, doi: 10.5455/msm.2013.25.255-258.
4. Eckstein R. Immunhämatologie und Transfusionsmedizin, Urban & Fischer, München, 2005.
5. Sandler SG, Chen LN, Flegel WA, Serological weak D phenotypes: a review and guidance for interpreting the RhD blood type using the RHD genotype. Br J Haematol. 2017; 179(1):10-19, doi: 10.1111/bjh.14757.
6. Aljuhaysh RM, El-Fetoh NMA, Alanazi MI, et al. Maternal-fetal Rhesus (Rh) factor incompatibility in Arar, northern Saudi Arabia. Electron Physician. 2017; 25; 9(12):5908-5913. doi: 10.19082/5908.
7. Bowman J. The management of hemolytic disease in the fetus and newborn. Semin Perinatol. 1997; 21(1):39-44.
8. Lawick S, Coberly EA, Lee LA, Johnson M, Eichbaum Q. Jk3 alloantibodies during pregnancy-blood bank management and hemolytic disease of the fetus and newborn risk. Transfusion. 2018; 58(5):1157-1162, doi: 10.1111/trf.14548.
9. American Academy of Pediatrics and American College of Obstetricians and Gynecologists, Prevention of Rh D Alloimmunization.
10. Lindenburg IT, van Kamp IL, Oepkes D. Intrauterine blood transfusion: current indications and associated risks. Fetal Diagn Ther. 2014; 36(4):263-71, doi: 10.1159/000362812.
11. Ghesquière L, et al. Management of red blood cell alloimmunization in pregnancy. J Gynecol Obstet Hum Reprod. 2018; 47(5):197-204, doi: 10.1016/j.jogoh.2018.02.001.
12. Nicolaides KH, Soothill PW, Clewell WH, Rodeck CH, Mibashan RS, Campbell S. Fetal haemoglobin measurement in the assessment of red cell isoimmunization (Level II-3). Lancet. 1988; 1:1073-5.
13. Moise KJ Jr. Management of rhesus alloimmunization in pregnancy. Obstet Gynecol. 2002; 100(3):600-11.
14. Gregory L. Rhesus and Other Fetomaternal Incompatibilities Emery and Rimoin’s Principles and Practice of Medical Genetics. In: Emery and Rimoin’s Principles and Practice of Medical Genetics, 6th edition. Academic Press, USA, 2013.
15. Shin JH, Lee JY, Kim JH, Kim HR, Lee JN.Screening and identification of unexpected red cell antibodies by simultaneous LISS/Coombs and NaCl/Enzyme gel methods. J Korean Med Sci. 2009; 24(4):632-5, doi: 10.3346/jkms.2009.24.4.632.
16. Wikman A, Jalkesten E, Ajne G, Höglund P, Mörtberg A, Tiblad E. Anti-D quantification in relation to anti-D titre, middle cerebral artery Doppler measurement and clinical outcome in RhD-immunized pregnancies. Vox Sang. 2018 Nov; 113(8):779-786, doi: 10.1111/vox.12716..
17. Kent J, Farrell AM, Soothill P. Routine administration of Anti-D: the ethical case for offering pregnant women fetal RHD genotyping and a review of policy and practice. BMC Pregnancy Childbirth. 2014; 14:87, doi: 10.1186/1471-2393-14-87.
18. Crowther C, Middleton P. Anti-D administration after childbirth for preventing Rhesus alloimmunization. Cochrane Database Syst Rev. 2000;(2): CD000021.
19. Knowles S. Blood transfusion: challenges and limitations. Transfusion Alternatives in Transfusion Medicine. 2007; 9. 2-9. 10.1111/j.1778-428X.2007.00062.x.
20. Rowley M, et al. Laboratory Aspects of Blood Transfusion. In: Dacie and Lewis Practical Haematology (Twelfth Edition), 2017.
21. Society for Maternal-Fetal Medicine (SMFM). Society for Maternal-Fetal Medicine (SMFM). Society for Maternal-Fetal Medicine (SMFM) Clinical Guideline #8: the fetus at risk for anemia – diagnosis and management. Am. J. Obstet. Gynecol. 2015 Jun; 212(6):697-710.
22. Troìa L, Al-Kouatly HB, McCurdy R, Konchak PS, Weiner S, Berghella V. The Recurrence Risk of Fetomaternal Hemorrhage. Fetal Diagn Ther. 2019; 45(1):1-12.
23. Kelly TF, Moore TR. Maternal Medical Disorders of Fetal Significance. In: Gleason C, Devaskar S. Avery’s Diseases of the Newborn (Ninth Edition). Saunders. 2012.
24. Khalil A, et al. Haematological Disorders. In: Coady AM, Bower S (ed.). Twining’s Textbook of Fetal Abnormalities (Third Edition). Elsevier. 2015.
25. Callum J, Barrett J. In: Hillyer CD (ed.). Transfusion Medicine, Blood Banking and Transfusion Medicine (Second Edition). Elsevier. 2007.
26. Ville Y. Invasive procedures in obstetrics. Ultrasound in Obstetrics and Gynaecology. 2009; 229-245.
27. Bowman JM. The management of Rh-isoimmunization. Obstet Gynecol. 1978; 52:1.
28. Nwogu LC, Moise KJ Jr, Klein KL, Tint H, Castillo B, Bai Y. Successful management of severe red blood cell alloimmunization in pregnancy with a combination of therapeutic plasma exchange, intravenous immune globulin, and intrauterine transfusion. Transfusion. 2018; 58(3):677-684. doi: 10.1111/trf.14453.