Aims. To demonstrate the coexistence of skin disorders and diabetes mellitus (DM) in women with poor obstetric history. Methodology. This retrospective study comprised 69 women having poor obstetric history and skin disorders. The patients were grouped as control group (51 women without type 2 DM, with 84 skin disorders in total) and study group (18 women with type 2 DM, with 30 skin disorders in total). Skin disorders were grouped as follows: herpes zoster, viral warts, fungal diseases, melanocytic nevi, bacterial infections, hidradenitis suppurativa, dermatitis, psoriasis, lichen planus, urticaria, hair and nail disorders, acneiform diseases, epidermal cyst, melanine hyperpigmentation, hypertrophic disorders of the skin, mycosis fungoides and vitiligo. Beksaç Obstetric Index (BOI), which is “(the number of living child + π/10)/gravida”, was used for the evaluation of obstetric history. Results. The rate of DM was found to be 26.1% in this specific study population. We have demonstrated multiple skin disorders in 50% (9/18) of DM (+) women, while this rate was 37.3% (19/51) in the DM (-) women (p=0.504). A total of 114 skin disorders in 69 women were evaluated, and the rates of the dermatitis (38.9% versus 19.6%) and acneiform diseases (27.8% versus 13.7%) were found to be more frequent in DM (+) patients compared to DM (-) women (p>0.05, for all). Conclusions. Skin disorders are easy to detect in medical examinations and can be used as a remark for the investigation of DM, especially in women with poor obstetric history.
Istoricul obstetrical sărac, tulburările dermatologice şi diabetul zaharat de tip 2
Poor obstetric history, skin disorders and type 2 diabetes mellitus
First published: 24 mai 2022
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/ObsGin.70.1.2022.6509
Abstract
Introduction
Pregestational type 2 diabetes mellitus (DM) is associated with adverse fetal and maternal outcomes(1). Studies suggest that optimal control of diabetes before and during pregnancy minimizes these risks(2,3). However, pregnancies in women with type 2 DM are still poorly planned and complicated by higher rates of perinatal morbidity/mortality and major congenital malformations(4). The skin is highly sensitive to autoimmune disorders, allergic problems, metabolic diseases, internal organ diseases, paraneoplastic syndromes, bacterial/viral infections, inflammation or epigenetic reasons, and skin problems might be the sign of these disorders which might also be risk factors for poor gestational outcome(5-8).
Skin disorders (cutaneous infection, dry skin, pruritus etc.) are usually neglected and frequently underdiagnosed in diabetic patients, who encounter a broad spectrum of disorders(9). The most common skin manifestations of DM were reported to be “dry skin”, xerosis and acquired ichthyosis, occurring predominantly on the shins and feet(10). Loss of hair over the legs and diabetic foot ulcer were reported to be cutaneous markers of DM(11). Diabetes mellitus is an interdisciplinary disorder and acanthosis nigricans, acral erythema and onychoschizia showed a significant correlation with age and disease duration, while knuckle pebbles, eczema, facial erythema and koilonychia were significantly correlated with glycated hemoglobin (HbA1c)(12).
The aim of this study was to demonstrate the coexistence of skin disorders and DM in women with poor obstetric history, and to determine whether co-occurrence of these comorbidities are risk factors for poor gestational outcome.
Patients/materials and method
This retrospective study involved 69 women with poor obstetric history and skin disorders. Patients were grouped as control group (84 skin disorders of 51 type 2 DM [-] women)) and study group (30 skin disorders of 18 type 2 DM [+] women). Poor obstetric history was defined as having miscarriage, fetal growth restriction, preterm birth, preeclampsia and stillbirth in previous gestations. Patients with skin disorders were examined and evaluated for the presence of carbohydrate metabolism disorders within a framework of pre-pregnancy care program. Necessary laboratory tests, including 50-gram glucose challenge test, were performed. DM is defined as chronic metabolic disorder characterized by persistent hyperglycemia. It may be due to impaired insulin secretion, resistance to peripheral actions of insulin, or both(13). Women with DM were referred to the endocrinology department for their managements.
Skin disorders were grouped according to their dermatological findings, into seventeen categories: herpes zoster, viral warts, fungal diseases, melanocytic nevi, bacterial infections, hidradenitis suppurativa, dermatitis, psoriasis, lichen planus, urticaria, hair and nail disorders, acneiform diseases, epidermal cyst, melanine hyperpigmentation, hypertrophic disorders of the skin, mycosis fungoides and vitiligo. Beksaç Obstetric Index (BOI), which is “(number of living child + π/10)/gravida”, was used for the evaluation of obstetric history(14). The study groups were compared in terms of demographic findings, BOI and DM.
Demographic and clinical data were obtained from the electronic database of Division of Perinatology (2016-2019). Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS, version 23). Groups were compared using the Yates’ Chi-square and Fisher’ exact tests; p-values below 0.05 were considered as statistically significant. This study was approved by the Local Ethic Committee (GO 19/1064). This study was based in accordance with the Declaration of Helsinki.
Results
A total of 69 women with poor obstetric history and skin disorders were evaluated in terms of the presence of DM within a framework of a special pre-pregnancy care program. The rate of the presence of DM was found to be 26.1% in this specific study population. We have demonstrated multiple skin disorders in 50% (9/18) of DM (+) group, while this rate was 37.3% (19/51) in the DM (-) group (p=0.504) – Table 1. Table 2 shows the frequencies of skin disorders in DM (+) women.
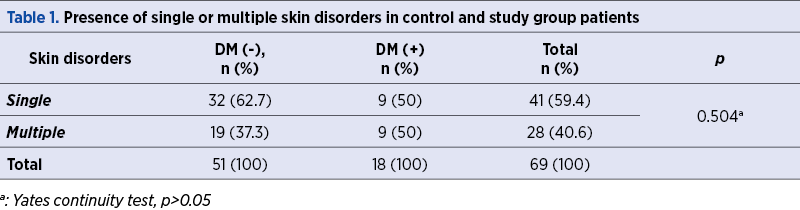
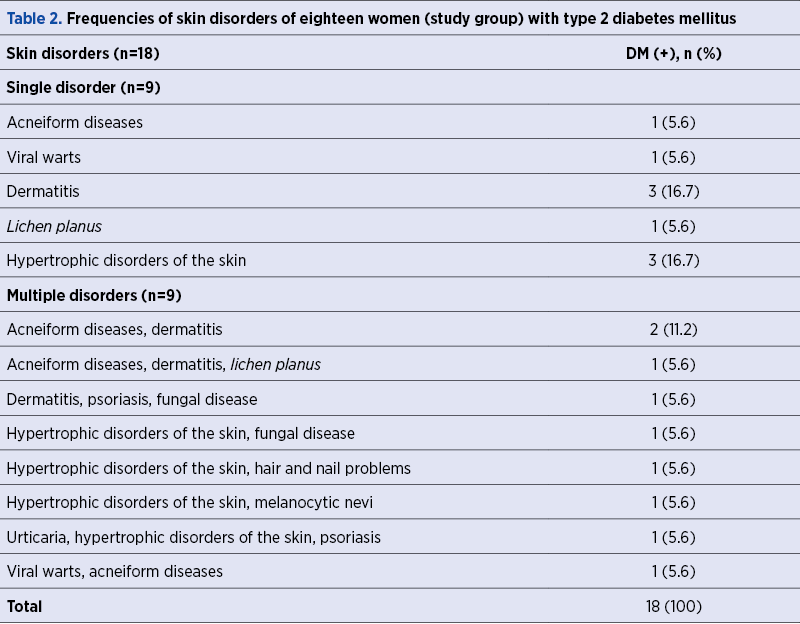
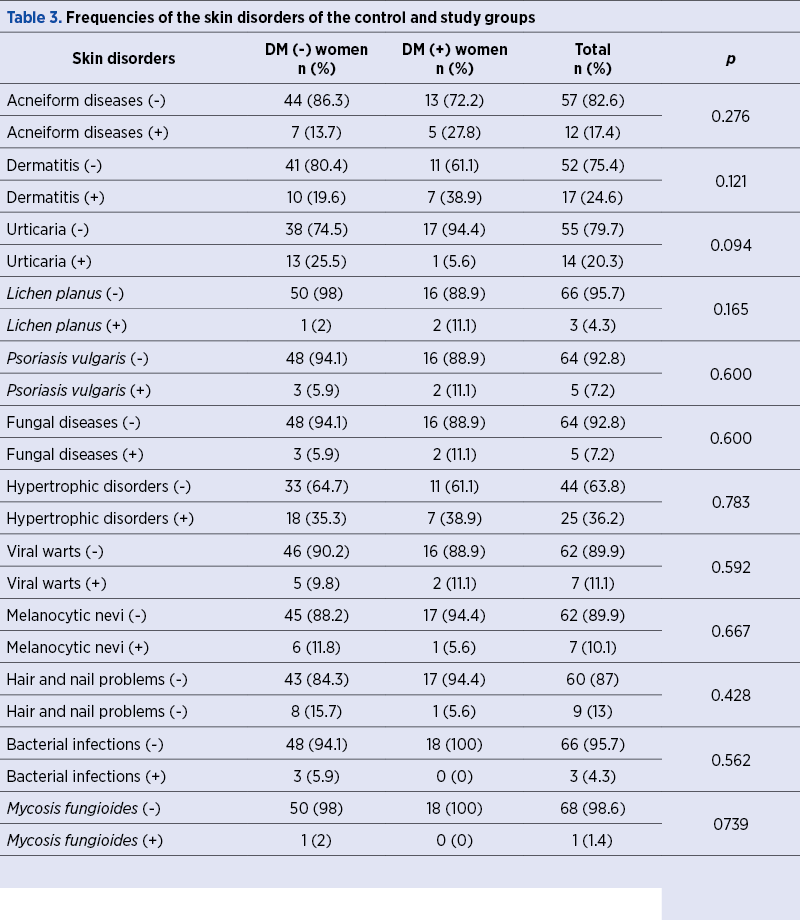
Table 3 shows the comparison of study and control groups in terms of the presence of skin disorders. A total of 114 skin disorders in 69 women were evaluated, and the rates of the dermatitis (38.9% versus 19.6%) and acneiform diseases (27.8% versus 13.7%) were found to be more frequent in DM (+) patients compared to controls, although this difference was not statistically significant (p>0.05, for all).
Discussion
The global DM prevalence in 2019 is estimated to be 9.3% and is expected to rise to 10.2% by 2030(15). It has also been reported that half billion people are living with DM worldwide, which means that over 10.5% of the world’s adult population currently have this condition(16). In this study, we have demonstrated that 26.1% of women with skin disorders and poor obstetric history had type 2 DM.
We previously reported that autoimmune disorders were statistically significantly more frequent in patients with poor gestational outcome and skin tags(7). Autoimmune disorders are also reported to be risk factors for placenta-related obstetric complications(17,18). Patients with skin diseases and poor obstetric history should be further evaluated in terms of the existence of inflammatory disorders which might be risk factors for adverse gestational outcome(8). Thus, preconception counseling is beneficial for women with poor obstetric history and skin problems(7,8). In this study, we have demonstrated that dermatitis and acneiform diseases were more frequent in women with poor obstetric history and DM. The rise in DM prevalence has lead to an increase in the number of pregnancies complicated by type 2 diabetes, and studies have shown risk of adverse outcome, including congenital malformation and perinatal morbidity/mortality(19,20).
Wide spectrum of skin disorders may occur as a result of DM and should be considered as a risk factor for poor gestational outcome(9-12). Thus, pre-pregnancy evaluation and management of DM (+) patients with skin disorders are especially important not to have obstetrical problems in their subsequent pregnancies. Several randomized clinical trials have demonstrated a beneficial effect of glycemia-lowering therapy on the outcomes in type 2 diabetes(21,22).
The main limitations of this study are the limited number of patients and its retrospective design. On the other hand, this study emphasizes the coexistence of skin disorders and DM in women with poor obstetric history.
In conclusion, skin disorders are easy to be detected in medical examinations and can be used as a signal for the investigation of DM especially in women with poor obstetric history. Pre-pregnancy counseling is important in the management of risky gestations, as well as for reducing perinatal morbidity and mortality.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
Darke J, Glinianaia SV, Marsden P, Bell R. Pregestational diabetes is associated with adverse outcomes in twin pregnancies: a regional register-based study. Acta Obstet Gynecol Scand. 2016;95(3):339-46. Doi: 10.1111/aogs.12825.
Clausen TD, Mathiesen E, Ekbom P, Hellmuth E, Mandrup-Poulsen T, Damm P. Poor pregnancy outcome in women with type 2 diabetes. Diabetes Care. 2005;28(2):323-8. Doi: 10.2337/diacare.28.2.323.
Boulot P, Chabbert-Buffet N, d’Ercole C, Floriot M, Fontaine P, Fournier A, Gillet JY, Gin H, Grandperret-Vauthier S, Geudj AM, Guionnet B, Hauguel-de-Mouzon S, Hieronimus S, Hoffet M, Jullien D, Lamotte MF, Lejeune V, Lepercq J, Lorenzi F, Mares P, Miton A, Penfornis A, Pfister B, Renard E, Rodier M, Roth P, Sery GA, Timsit J, Valat AS, Vambergue A, Verier-Mine O; Diabetes and Pregnancy Group, France. French multicentric survey of outcome of pregnancy in women with pregestational diabetes. Diabetes Care. 2003;26(11):2990-3. Doi: 10.2337/diacare.26.11.2990.
Beksac B, Donmez HG. Association of bacterial vaginosis and skin disorders in patients with autoimmune antibody positivity. Gynecol Obstet Reprod Med. 2021;27(2):150-3. Doi: 10.21613/GORM.2020.1103
Hu Y, Zhu Y, Lian N, Chen M, Bartke A, Yuan R. Metabolic syndrome and skin diseases. Front Endocrinol (Lausanne). 2019;10:788. Doi: 10.3389/fendo.2019.00788.
Beksac B, Donmez HG, Cagan M, Unal C, Fadiloglu E, Beksac MS. Acrochordons and autoimmunity: Significance of preconceptional counceling. Hum Antibodies. 2020;28(4):335-339. Doi:103233/HAB-200426.
Beksac B, Donmez HG, Beksac MS. Association of high levels of C-reactive protein with skin disorders in women having poor obstetric history. Obstetrica şi Ginecologia. 2021;69(2):58-61. Doi: 10.26416/obsgin.69.2.2021.4975.
De Macedo GM, Nunes S, Barreto T. Skin disorders in diabetes mellitus: an epidemiology and physiopathology review. Diabetol Metab Syndr. 2016;8(1):63. Doi: 10.1186/s13098-016-0176-y.
Piérard GE, Seité S, Hermanns-Lê T, Delvenne P, Scheen A, Piérard-Franchimont C. The skin landscape in diabetes mellitus. Focus on dermocosmetic management. Clin Cosmet Investig Dermatol. 2013;6:127-35. Doi: 10.2147/CCID.S43141.
Timshina DK, Thappa DM, Agrawal A. A clinical study of dermatoses in diabetes to establish its markers. Indian J Dermatol. 2012;57(1):20-5. Doi: 10.4103/0019-5154.92671.
Azizian Z, Behrangi E, Hasheminasabzavareh R, Kazemlo H, Esmaeeli R, Hassani P. Prevalence Study of Dermatologic Manifestations among Diabetic Patients. Adv Prev Med. 2019;2019:5293193. Doi: 10.1155/2019/5293193.
Goyal R, Jialal I. Diabetes Mellitus Type 2. [Updated 2021 Sep 28]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK513253/
Beksaç MS, Aydin E, Turğal M, Karaağaoğlu E. An Obstetrics Index for the Assessment of Risk Levels of “High Risk Pregnancy” Groups. Gynecol Obstet Reprod Med. 2015;21:10-3.
Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R; IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. Doi: 10.1016/j.diabres.2019.107843.
Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ. IDF diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. Doi: 10.1016/j.diabres.2021.109119.
Beksac MS, Tanacan A, Ozten G, Cakar AN. Low-dose low-molecular-weight heparin prophylaxis against obstetrical complications in pregnancies with metabolic and immunological disorder-associated placental inflammation. J Matern Fetal Neonatal Med. 2020 Apr 30;1-8. Doi: 10.1080/14767058.2020.1760834.
Tanacan A, Beksac MS, Orgul G, Duru S, Sener B, Karaagaoglu E. Impact of extractable nuclear antigen, anti-double stranded DNA, antiphospholipid antibody, and anticardiolipin antibody positivity on obstetrical complications and pregnancy outcomes. Hum Antibodies. 2019;27(2):135-41. Doi: 10.3233/HAB-180359.
Temple R, Murphy H. Type 2 diabetes in pregnancy - An increasing problem. Best Pract Res Clin Endocrinol Metab. 2010;24(4):591-603. Doi: 10.1016/j.beem.2010.05.011.
Lin SF, Chang SH, Kuo CF, Lin WT, Chiou MJ, Huang YT. Association of pregnancy outcomes in women with type 2 diabetes treated with metformin versus insulin when becoming pregnant. BMC Pregnancy Childbirth. 2020;20(1):512. Doi: 10.1186/s12884-020-03207-0.
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577-89. Doi: 10.1056/NEJMoa0806470.
Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, Rossing P, Tsapas A, Wexler DJ, Buse JB. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669-701. Doi: 10.2337/dci18-0033.
Articole din ediţiile anterioare
Pregnancy outcome at very advanced maternal age: the experience of a level 2 center
The reproductive age of individuals in developed countries has increased in recent decades. However, as age increases, fertility tends to decrease,...