Postpartum hemorrhage (PPH) is still a significant cause of death worldwide. This condition is classically defined as a blood loss of 500 ml or more within 24 hours after birth. Their most common causes are uterine atony, placental retention, coagulation disorders or genital trauma, and the management depends on each cause. PPH remains the leading cause of maternal mortality in low-income countries and stands out as the primary cause of nearly one quarter of all maternal deaths globally. Most deaths resulting from postpartum hemorrhage take place during the first 24 hours after birth, and the majority of these could be prevented through prophylactic uterotonics during the third stage of labor and by timely and appropriate management. Postpartum hemorrhage can occur in patients without priorly known risk factors for bleeding. The proper management of postpartum hemorrhage implies rapid diagnosis and treatment. The four Ts mnemonic can be used to diagnose and cure the four most common causes of postpartum hemorrhage: uterine atony (Tone); laceration, hematoma, inversion, rupture (Trauma); retained tissue or invasive placenta (Tissue); and coagulopathy (Thrombin). Fast-diagnosing team-based care significantly reduces the morbidity and mortality associated with postpartum hemorrhage, no matter the cause. This article plans to provide a scheme for the strategic policy and steps needed to ensure effective interventions in all PPH cases.
Managementul hemoragiei post-partum, în funcţie de cauza de bază
Postpartum hemorrhage management according to the underlying cause
First published: 30 decembrie 2023
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/ObsGin.70.4.2022.7490
Abstract
Rezumat
Hemoragia post-partum (HPP) continuă a fi o cauză importantă de deces la nivel mondial. HPP este definită în mod clasic ca o pierdere de sânge de 500 ml sau mai mult în primele 24 de ore după naştere. Cele mai frecvente cauze ale sângerării sunt atonia uterină, retenţia placentară, tulburările de coagulare sau traumatismele genitale, iar managementul depinde de fiecare cauză. HPP rămâne principala cauză a mortalităţii materne în ţările cu venituri mici şi se remarcă drept cauza principală a aproape un sfert din toate decesele materne la nivel global. Majoritatea deceselor rezultate în urma HPP au loc în primele 24 de ore după naştere. Majoritatea acestora ar putea fi prevenite prin administrarea profilactică de uterotonice în timpul celei de-a treia etape a travaliului şi printr-un management adecvat instituit în timp util. Hemoragia post-partum poate apărea la paciente fără factori de risc pentru sângerare cunoscuţi anterior. Gestionarea corectă a hemoragiei post-partum implică un diagnostic şi un tratament rapid. Mnemonicul celor patru „T” poate fi folosit pentru a diagnostica şi vindeca cele mai frecvente patru cauze ale hemoragiei post-partum (atonie uterină [ton]; laceraţie, hematom, inversare, ruptură [traumă]; ţesut reţinut sau placentă invazivă [ţesut] şi coagulopatie [trombină]). Alcătuirea unei echipe care să stabilească diagnosticul corect rapid reduce semnificativ morbiditatea şi mortalitatea asociate cu hemoragia postpartum, indiferent de cauză. Acest articol intenţionează să ofere o schemă pentru politica strategică şi paşii necesari care să asigure intervenţii eficiente pentru toate cazurile de HPP.
Introduction
“She died giving birth to a baby.” These terrible words have resonated over the years, and bleeding probably killed more women than any other pregnancy complication in human history. Every year, approximately 150,000 maternal deaths worldwide are caused by obstetric hemorrhage(1,2). The world’s leading cause of pregnancy-related deaths is considered to be postpartum hemorrhage (PPH)(3).
Women pregnant beyond 20 weeks are exposed to the risk of PPH and the complications that arise from it. In the developed world, mortality rates have fallen sharply, but elsewhere postpartum hemorrhage remains a leading cause of death(4). Postpartum hemorrhage with a blood loss higher than 500 ml has a rate of 10.8%, while PPH with a blood loss higher than 1000 ml has a rate of 2.8%(5).
Bleeding that occurs in the first 24 hours after birth is called primary or early PPH. Between 24 hours and 12 weeks, the bleeding is called secondary or late PPH.
The definition of postpartum hemorrhage is complex. The bleeding may not be visible, or the collected blood may be mixed with the amniotic fluid(6). PPH is described as a blood loss of more than 1000 ml after a caesarean delivery or more than 500 ml after vaginal birth(7,8).
The diagnosis may be delayed in symptomatic women when no bleeding is observed, such as after closing the abdomen at a caesarean delivery or intraabdominal bleeding after a vaginal birth(6).
In developing countries, the increased frequency of postpartum hemorrhage is probably reflected by the absence of a wide availability of drugs used in the active management of the third stage(9).
There is strong evidence that obstetricians often underestimate blood loss at birth. According to traditional definitions, at least half of births would have postpartum hemorrhages. Perhaps a better description of PPH would include sufficient blood loss to cause symptoms of hypovolemia, a 10% decrease in hematocrit after birth, or requiring a blood transfusion(10).
The physiologic mechanism of PPH
The term blood flow to the pregnant uterus is 800-1000 ml/min, explaining why large amounts of blood can be lost quickly. Maternal exsanguination may occur fast when the mechanisms to reduce blood loss are inefficient. The uterus contracts after the delivery of the placenta. Because myometrial fibers contract in different directions, the contraction of these fibers clogs the blood vessels, preventing heavy bleeding. This contraction is the major mechanism for hemostasis after birth (rather than clot formation or platelet aggregation). If the uterus is well contracted immediately after birth and the bleeding begins, it is probably the consequence of a laceration or injury to the genital tract. Primary HPP management must first ensure the contraction of the uterus and then identify and repair any lesions of the genital tract. The volume of maternal blood increases by 40% to 50% during pregnancy, because there increase both the plasma volume and the mass of the red blood cells. These natural changes appear to protect the mother from the consequences of bleeding during and after birth. After delivery, a woman may lose up to 20% of her blood volume before clinical signs become apparent. For example, when a volume reduction occurs in preeclampsia, women may be more sensitive to the effects of blood loss at birth, and it can be decompensated faster(11).
PPH causes
There are multiple causes of postpartum hemorrhage. In clinical practice, they are called the “four Ts”: tone (uterine atony), tissue (abnormal placental implantation or retained placenta), trauma (lacerations of birth canal, uterine rupture, cervical laceration, uterine inversion), and thrombin (coagulopathy)(12-14). After the delivery of the placenta, there is a possibility of inadequate myometrial contraction, which is called uterine atony, resulting in excessive blood loss. This complication occurs in 1 in 20 births(15).
At caesarean delivery, the lateral extension of the uterine incision causes bleeding. The lateral extension may result from the spontaneous rupture of an edematous lower uterine segment, from an incision made too low or insufficiently curved, or from the birth of the fetus through a too-small incision(16).
Risk factors
Pregnant women may be considered at risk if they have prolonged, difficult labor (fatigue due to prolonged labor leads to insufficient myometrial contraction) or they have chorioamnionitis.
The risk factors for postpartum hemorrhage are: caesarean section, anterior PPH, abruptio placentae, overdistension of the uterus (polyhydramnios, multifetal pregnancy, macrosomia, polyhydramnios, or fetal abnormality – for example, severe hydrocephalus), general anesthesia, therapy with anticoagulants, multiparity, a long time use of oxytocin, stillbirth, instrumental delivery, advanced maternal age.
In the placenta expulsion, three interventions are involved: uterotonics, immediate umbilical cord clamping, and controlled cord traction(21,23).
Prevention
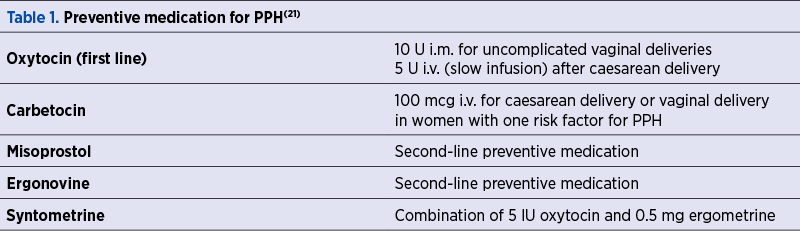
Regarding PPH prevention strategies, there are no specific recommendations for the third stage of labor (Table 1)(21).
One study by Shahyd et al.(15) found that the administration of tranexamic acid before a caesarean section significantly reduced blood loss during surgery.
However, the same study showed that blood loss was not reduced after the operation. The use of tranexamic acid was not followed by side effects(15).
Fibrinolysis caused by the activation of the fibrinolytic system at the time of placental delivery is inhibited by tranexamic acid. Movafegh et al. demonstrated that 10 mg/kg o f tranexamic acid administered intravenously 20 minutes before the skin incision at caesarean section was followed by a reduction in bleeding during and after surgery(22).
Uterine atony is the most common cause of PPH (about 80% of postpartum hemorrhage cases)(16). A diagnosis of atony is usually made when the uterus does not become retracted after uterine massage and oxytocin. Atonia may or may not be associated with retained tissue. Placental disorders such as disorders in the adherence of the placenta, placenta praevia, placental abruption, retained products of conception, and uterine inversion lead to postpartum hemorrhage because they prevent effective uterine contraction. The most common risk factors associated with atony are previous PPH and prolonged labor, but many other risk factors have been observed(17). The diagnosis of uterine atony is made by bimanual palpation of the uterus. The initial management is done by massaging the uterus and by intravenous administration of oxytocin(18).
Sir Henry Dale was the first to discover oxytocin in 1909. Oxytocin is the first-line drug used in the prevention and treatment of postpartum hemorrhage. The paraventricular nucleus of the hypothalamus produces it, and then the oxytocin is released by the posterior pituitary gland(24). Myometrial contractility is stimulated by oxytocin by increasing the concentration of calcium in cells, which binds to calmodulin, activating myosin light-chain kinase – the primary mechanism of action of the uterine smooth muscle(25,26). A non-pregnant uterus has no oxytocin receptors. Oxytocin receptors are present in the myometrium and appear from 13 weeks of gestational age, their distribution being uneven. The fundus of the uterus contains most of the receptors, their number decreasing towards the lower segment and the cervix(27).
It is commonly administered intravenously, providing a more precise and rapid response. The purpose is to ensure an accurate dose and fast ending of administration if an adverse reaction occurs. This drug has an immediate onset of action if the administration is intravascular(28). During caesarean delivery, 10 units of intramuscular injection are recommended once the placenta is delivered. Oxytocin is released into the extracellular space after being absorbed, and does not attach to plasma proteins. In plasma, it takes around 20 to 30 minutes to reach a steady-state concentration and about 40 minutes to reach a maximum concentration(29). The dose and pace of administration can potentially cause harmful cardiovascular adverse effects. Cardiovascular collapse and even death have occurred due to rapid intravenous bolus doses of up to five units(30).
The dose, timing and rate of oxytocin administration during caesarean delivery are still unknown. Kovecheva and colleagues employed a “rule of threes” algorithm in randomized, double-blind research to reduce the dose-related and rate-related side effects of oxytocin by using a standardized technique of giving oxytocin during elective caesarean deliveries. The protocol begins with 3 units of oxytocin administered over 5 seconds after the fetus is delivered, and uterine tone is measured every 3 minutes. If there is insufficient tone after each 3 minutes, additional 3 units of oxytocin are given. A second-line uterotonic drug is advised if a third bolus of oxytocin is given for inadequate tone and uterine atony persists(31).
The side effects include hemodynamic instability, like hyportension, tachycardia, myocardial ischemia and arrhythmias, and nausea, vomiting, headache and flushing. Hypotension and tachycardia are the most common side effects. Because of its structural resemblance to vasopressin, excessive doses of oxytocin can cause water intoxication, hyponatremia, convulsions and coma(32,33).
In smooth muscle, ergot alkaloids act as agonists for serotonergic receptors, weak antagonists for dopaminergic receptors, and partial agonists for alpha-adrenergic receptors. They cause persistent uterine contractions by inducing fast rhythmic uterine contractions (spasm and tetany)(34).
The American College of Obstetricians and Gynecologists suggests methylergonovine as a second-line uterotonic for unmanageable uterine atony(35).
The medications are commonly given intramuscularly at a dose of 0.2 mg in the United States of America. The onset occurs within 2-3 minutes, and the uterotonic effects extend for several hours. In Canada, the medications are given as a slow intravenous (i.v.) bolus over 1 minute, despite the fact that the US Food and Drug Administration advises against it, due to the risk of acute hypertension. Preeclampsia, various hypertension illnesses, peripheral vascular disease and ischemic heart disease are all relative contraindications to using ergot alkaloids. Ergot alkaloids are processed by cytochrome CYP3A4 and should not be taken with medications that block this isoenzyme, such as human immunodeficiency virus protease or reverse transcriptase inhibitors, macrolide antibiotics and azole antifungals. Vasospasm has been linked to limb and brain ischemia(36,37).
Hypertension, myocardial ischemia, infarction from coronary vasospasm, cerebrovascular accidents, seizures and death are serious side effects in rare cases(38).
Acute coronary syndrome or infarction is more likely in patients with coronary artery disease or risk factors for coronary artery disease. Smoking, obesity, diabetes and high cholesterol are all risk factors(39).
Carbetocin is a synthetic oxytocin analog that works by attaching to oxytocin receptors to generate uterine contractions(40).
The activity begins 2 minutes after intravenous or intramuscular (i.m.) delivery, and the half-life is 4-10 times higher than that of oxytocin (40 minutes). It permits a bolus dose to be given without requiring a maintenance infusion. Carbetocin has a similar adverse effect profile as oxytocin, and a prophylactic dose of 100 µg is clinically indicated(41).
Su et al. found evidence suggesting that 100 µg of intravenous carbetocin is more beneficial than oxytocin at preventing postpartum hemorrhage in women having caesarean births. However, more research is needed to confirm this discovery. In preventing PPH for women who have vaginal births, carbetocin is related to less blood loss than syntometrine and has fewer side effects. More research is required to determine the cost-effectiveness of carbetocin as a uterotonic agent(42).
A study by Cho et al. found that, in extensive hemorrhages refractory to uterotonics, intrauterine balloon tamponade should be explored as a last resort(43).
In another study by Vitthala et al., in addition to oxytocics, Bakri balloon tamponade can be used to decrease bleeding caused by uterine atony. The Bakri tamponade balloon is 58 cm long and constructed of silicone, making it suitable for latex-allergic patients(47).
Bakri pioneered intrauterine balloon tamponade to treat obstetric bleeding during caesarean birth in 1992(44).
Previously, uterine tamponade was performed by packing the uterus with cotton gauze, which had several drawbacks. Uterine packing is a time-consuming procedure that might result in uterine damage and endometritis(45).
The Bakri balloon is an intrauterine device used to lessen or manage PPH when conservative treatment is needed temporarily. It looks like an effective therapy option for acute postpartum hemorrhage that has resisted medical treatment and requires little training. The device comprises a silicone balloon attached to a silicone catheter. The balloon is filled with liquid and put into the uterine cavity, it conforms to the contour of the cavity and stops the bleeding. The blood drains into the catheter’s core lumen, and blood loss can then be measured(46).
The catheter is introduced into the lower uterine region. The balloon is filled with up to 500 mL of sterile solution and left in place for 8 to 48 hours before gradually deflating and removing the catheter(48).
Women with anatomic uterine cavity changes, suspected uterine, cervical or vaginal infection, or with uterine rupture should avoid using the Bakri balloon(49).
It is less expensive than the B-Lynch suture since it does not require a laparotomy or trained senior staff to apply. Due to the possibility of uterine muscle necrosis under the suture, B-Lynch suture may impact future fertility(50,51).
Compression sutures are most commonly used to treat hemorrhage caused by uterine atony, although they can also be utilized whenever uterine compression is needed to reduce uterine bleeding.
The insertion of uterine compression sutures 2-6 hours after birth is related to an increased risk of hysterectomy(65).
B-Lynch et al. describe a compression suture that is particularly useful in cases where a lower transverse caesarean section has been performed(66).
According to Cetin et al., the Hayman uterine compression suture and the Bakri balloon had the same hemostatic efficiency in patients with atonic hemorrhage. The treatment should be chosen depending on various parameters, such as the severity of the bleeding and the surgeon’s experience(64).
Genital tract trauma, uterine rupture and uterine inversion
Childbirth or various interventions can lead to cervical and vaginal lacerations. They may not be noticed until excessive postpartum vaginal bleeding requires examination of the lower genital tract, including inspection for vaginal and vulvar hematomas. Lacerations of the uterine body may be complete transmiometric ruptures or incomplete lacerations of the internal myometrium(18).
Premature labor, surgical vaginal birth and cerclage are all risk factors for major cervical lacerations(52).
Using an absorbable suture, repair highly bleeding vaginal and cervical lacerations. Due to issues with exposure and vision, starting a suture line at the apex of the tear might be challenging. In such circumstances, start the suture line at the distal end of the laceration and sew toward the apex, pulling the lacerated tissue toward the surgeon with the stitch.
Hematomas in the vaginal canal should not be drained unless they are growing. Because it is sometimes difficult to locate and ligate bleeding arteries in a fresh vaginal sulcus hematoma, attempts at surgical drainage can result in significant extra blood loss. If a stable hematoma develops infected or pain is not effectively eased with painkillers, it may be drained later.
Sutures should not be inserted cephalad to the fornix, because ureteral ligation can occur. Perform a laparotomy if there is an extension high in the vagina, maybe extending into the cardinal ligament.
Uterine rupture is an uncommon finding. It is crucial to check for it in individuals who exhibit indications of PPH and have had a trial of labor after a previous caesarean delivery. Unscarred uterine rupture is uncommon, but it can happen if labor is induced or augmented, and even more rarely, following an instrumental delivery. Despite the use of uterotonic medications, uterine rupture is commonly marked by discomfort and continuous vaginal bleeding after birth. Even mild hemodynamic instability in any postpartum patient – whether or not she had bleeding – should urge uterine rupture and intraabdominal bleeding to be considered. If the rupture continues into the bladder, hematuria may develop. Intraabdominal bleeding should also be considered if maternal symptoms of hypovolemia appear to be out of proportion to the observed blood loss and abdominal distention. The aperture, which might be anterior, fundal, posterior or lateral, can be shown by palpating the uterine cavity. An abdominal ultrasound may detect blood and/or an expansive ligament hematoma.
If a uterine rupture is discovered, the best surgical treatment is a hysterectomy. However, uterine repair may be achievable depending on the patient’s plans for future pregnancies, the extent of uterine damage, hemodynamic stability and the surgeon’s abilities(53).
Retained placenta and clots
Placental fragments stop adequate uterine contractions, and this incident can cause uterine atony and postpartum hemorrhage(19). If feasible, remove any remaining placental pieces or fetal membranes manually or with ring forceps(54). Ultrasound examination can aid in diagnosing and guide the removal of retained tissue. If manual removal fails to limit hemorrhage, curettage with a 16 mm suction tube or a big blunt curette is used(53).
Uterine inversion
Before placental separation, uterine inversion can occur due to the solid traction on the cord. Blood loss is disproportionate to the shock and, because of that, it can become life-threatening(19).
When the uterine fundus collapses into the endometrial cavity, the uterus is turned partially or entirely inside out, resulting in uterine inversion(55).
Following a vaginal or caesarean delivery, puerperal uterus inversion can occur, including inversion through the hysterotomy incision(56,57).
The treatment of acute uterine inversion should begin as soon as possible. Uterine relaxation is required to replace the uterine fundus. It is mandatory to discontinue uterotonic medications. Additional obstetricians and anesthesiologists are needed for the following steps.
Attempt to return the inverted uterus to its natural position as soon as possible. The standard procedure is to place a hand within the vagina, and pulling the fundus toward the umbilicus along the long axis of the vagina. If manual uterine replacement fails, the patient may need to be brought to the operating room. Set up proper intravenous access and resuscitation with fluids and blood products(58).
More blood is lost when the placenta is removed before the uterus is replaced, which might be dangerous. It is important to remember that the placenta should not be delivered until the uterus has been replaced(59,60).
After the uterus has been replaced, the most conservative strategy is to wait for the placenta to separate naturally. Atony is prevalent after inversion correction. Following a successful placental removal, uterotonic drugs are used to promote myometrial contraction and sustain uterine involution, preventing reinversion and lowering the risk of bleeding. If the patient does not have a penicillin allergy, a single dosage of a first-generation cephalosporin can be given for endometritis prevention(61-63).
Coagulation disorders
Women with von Willebrand disease are particularly at risk for postpartum hemorrhage, as von Willebrand factor levels, which usually rise during pregnancy, drop rapidly after birth. Amniotic fluid embolism, placental abruption, preeclampsia with severe features or HELLP syndrome can lead to acute acquired coagulopathies(20).
Massive hemorrhage related to obstetrical or surgical bleeding is the most common cause of coagulopathy during postpartum hemorrhage(67).
In coagulopathy, plasma is utilized to replace coagulation factors.
When specific factor concentrations are unavailable, cryoprecipitate replaces fibrinogen and cures single-factor shortages. Fibrinogen, factor VIII, factor XIII and von Willebrand factor are all present in cryoprecipitate. Because cryoprecipitate is acellular, compatibility testing is not required.
Platelet transfusions help patients with thrombocytopenia or platelet dysfunction achieve primary hemostasis (congenital, metabolic or medication-induced).
Recombinant activated factor VIIa has been used to treat severe postpartum hemorrhage, mainly in cases where transfusions have failed(68).
Conclusions
Postpartum hemorrhage remains nowadays a terrifying complication that may intervene in each birth. Whenever the medical team attending the labor is adequately prepared to diagnose a postpartum hemorrhage quickly and to intervene correctly, the patient has a good chance for a full recovery. We must remember at any time that there is no such notion as excessive education and preparation to care for a laboring woman.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
-
Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet (London, England). 2006 Apr;367(9516):1066–74.
-
AbouZahr C. Global burden of maternal death and disability. Br Med Bull. 2003;67:1–11.
-
Organization WH. Maternal mortality in 2000 : estimates developed by WHO, UNICEF and UNFPA [Internet]. Geneva PP - Geneva: World Health Organization; Available from: https://apps.who.int/iris/handle/10665/42930
-
Centers for Disease Control and Prevention [Internet]. Available from: https://www.cdc.gov/reproductivehealth/maternal-mortality/pregnancy-mortality-surveillance-system.htm
-
Frigo MG, Agostini V, Brizzi A, Ragusa A, Svelato A. Practical approach to transfusion management of postpartum haemorrhage. Transfus Med. 2021 Feb;31(1):11–5.
-
Anger H, Durocher J, Dabash R, Winikoff B. How well do postpartum blood loss and common definitions of postpartum hemorrhage correlate with postpartum anemia and fall in hemoglobin? PLoS One. 2019;14(8):e0221216.
-
Baskett TF. Complications of the third stage of labour. In: Essential Management of Obstetrical Emergencies. 3rd ed. Bristol, England, Clinical Press, 1999, p. 196–201.
-
Sentilhes L, Vayssière C, Deneux-Tharaux C, Aya AG, Bayoumeu F, Bonnet M-P, et al. Postpartum hemorrhage: guidelines for clinical practice from the French College of Gynaecologists and Obstetricians (CNGOF): in collaboration with the French Society of Anesthesiology and Intensive Care (SFAR). Eur J Obstet Gynecol Reprod Biol. 2016Mar;198:12–21.
-
WHO Recommendations for the Prevention and Treatment of Postpartum Haemorrhage. Geneva: World Health Organization; 2012.
-
ACOG Practice Bulletin: Clinical Management Guidelines for Obstetrician-Gynecologists Number 76, October 2006: postpartum hemorrhage. Obstet Gynecol. 2006 Oct;108(4):1039–47.
-
Kominiarek MA, Kilpatrick SJ. Postpartum hemorrhage: a recurring pregnancy complication. Semin Perinatol. 2007;31(3):159–66.
-
Lindquist JD, Vogelzang RL. Pelvic Artery Embolization for Treatment of Postpartum Hemorrhage. Semin Intervent Radiol. 2018;35(1):41–7.
-
Katz D, Beilin Y. Management of post-partum hemorrhage and the role of the obstetric anesthesiologist. J Matern Fetal Neonatal Med. 2021;34(9):1487-1493.
-
Giurazza F, Corvino F, Paladini A, et al. Uterine Arteriovenous Fistula with Concomitant Pelvic Varicocele: Endovascular Embolization with Onyx-18®. Case Rep Vasc Med. 2017;2017:3548271.
-
Shahid A, Khan A. Tranexamic acid in decreasing blood loss during and after caesarean section. J Coll Physicians Surg Pak. 2013 Jul;23(7):459–62.
-
Reale SC, Easter SR, Xu X, Bateman BT, Farber MK. Trends in Postpartum Hemorrhage in the United States From 2010 to 2014. Anesth Analg. 2020;130(5):e119–22.
-
Ende HB, Lozada MJ, Chestnut DH, Osmundson SS, Walden RL, Shotwell MS, et al. Risk Factors for Atonic Postpartum Hemorrhage: A Systematic Review and Meta-analysis. Obstet Gynecol. 2021;137(2):305–23.
-
Conrad LB, Groome LJ, Black DR. Management of Persistent Postpartum Hemorrhage Caused by Inner Myometrial Lacerations. Obstet Gynecol. 2015;126(2):266–9.
-
Oyelese Y, Ananth CV. Postpartum Hemorrhage: Epidemiology, Risk Factors, and Causes. Clin Obstet Gynecol [Internet]. 2010;53(1). Available from: https://journals.lww.com/clinicalobgyn/Fulltext/2010/03000/Postpartum_Hemorrhage__Epidemiology,_Risk_Factors,.16.aspx
-
Corvino F, Giurazza F, Vallone M, Mosca S, Fischer MJ, Corvino A, et al. Postpartum Hemorrhage: Rescue. Semin Ultrasound CT MR. 2021 Feb;42(1):75–84.
-
Dahlke JD, Mendez-Figueroa H, Maggio L, Hauspurg AK, Sperling JD, Chauhan SP, et al. Prevention and management of postpartum hemorrhage: a comparison of 4 national guidelines. Am J Obstet Gynecol. 2015 Jul;213(1):76.e1-76.e10.
-
Movafegh A, Eslamian L, Dorabadi A. Effect of intravenous tranexamic acid administration on blood loss during and after cesarean delivery. Int J Gynaecol Obstet. 2011;115(3):224-226.
-
Society of Obstetrics and Gynecology of Canada. Postpartum hemorrhage. ALARM Manual. 15th Ed., 2008.
-
Dale HH. The action of extracts of the pituitary body. Biochem J. 1909;4:427–47.
-
Dyer RA, Van Dyk D, Dresner A. The use of uterotonic drugs during caesarean section. Int J Obstet Anesth. 2010;19:313–9.
-
Dyer RA, Butwick AJ, Carvalho B. Oxytocin for labour caesarean delivery: implications for the the anaesthesiologist. Curr Opin Anesthesiol. 2011;24(3):255–61.
-
Arais F. Pharmacology of oxytocin and prostaglandins. Clin Obstet Gynecol. 2000;43(3):455–68.
-
Oladapo OT, Okusanya BO, Abalos E. Intramuscular versus intravenous prophylactic oxytocin for the third sate of labour. Cochrane Database Syst Rev. 2012;(2):CD009332.
-
Dawood MY, Ylikorkala O, Trivedi D, et al. Oxytocin levels and disappearance rate and plasma follicle-stimulant hormone and luteinizing hormone after oxytocin infusion in men. J Clin Endocrinol Metab. 1980;50(2):397–400.
-
Thomas JS, Koh SH, Cooper GM. Haemodynamic effects of oxytocin given as IV bolus or infusion on women undergoing caesarean section. Br J Anaesth. 2007;98(1):116–9.
-
Kovacheva FP, Soens MA, Tsen LC. A randomized, double-blinded trial of a “rule of threes” algorithm versus continuous infusion of oxytocin during elective cesarean delivery. Anesthesiology. 2015;123(1):92–9.
-
Pinder AJ, Dresner C, Calow C, et al. Haemodynamic changes caused by oxytocin during caesarean section under spinal anesthesia. Int J Obstet Anesth. 2002;11(3):156–9.
-
Begrum D, Lonnee H, Hakli TF. Oxytocin infusion: acute hyponatremia, seizures, and coma. Acta Anasthesiol Scand. 2009;53(6):826–7.
-
den Hertog CE, de Groot AN, van Dongen PW. History and use of oxytocics. Eur J Obstet Gynecol Reprod Biol. 2001;94(1):8-12.
-
American College of Obstetricians and Gynecologists. ACOG Practice Bulletin: Clinical Management Guidelines for Obstetrician-Gynecologists Number 76, October 2006: postpartum hemorrhage. Obstet Gynecol. 2006;108(4):1039–47.
-
Methergine (package insert). https://www.accessdata.fda.gov/drugsatfda_docs/ label/2012/006035s078lbl.pdf; 2012. Accessed: February 28, 2021.
-
Leduc D, Senikas V, Lalonde AB. Society of Obstetricans and Gynaecologists of Canada. No. 235-Active management of the third stage of labour: Prevention and treatment of postpartum hemorrhage. J Obstet Gynaecol Can. 2018;40:e841–e855.
-
Vallera C, Choi LO, Cha CM, Hong RW. Uterotonic medications: oxytocin, methylergonovine, carboprost, misoprostol. Anesthesiol Clin. 2017;35:207–219.
-
Bateman BT, Huybrechts KF, Hernandez-Diaz S, et al. Methylergonovine maleate and the risk of myocardial ischemia and infarction. Am J Obstet Gynecol. 2013; 209(5):459.e1–13.
-
Meshykhi LS, Nel MR, Lucas DN. The role of carbetocin in the prevention and management of postpartum haemorrhage. Int J Obstet Anesth. 2016;28:61–69.
-
Leduc D, Senikas V, Lalonde AB. Society of Obstetricans and Gynaecologists of Canada. No. 235-Active management of the third stage of labour: Prevention and treatment of postpartum hemorrhage. J Obstet Gynaecol Can. 2018;40:e841–e855.
-
Su LL, Chong YS, Samuel M. Carbetocin for preventing postpartum haemorrhage. Cochrane Database Syst Rev. 2012;(4):CD005457.
-
Cho HY, Park YW, Kim YH, Jung I, Kwon JY. Efficacy of Intrauterine Bakri Balloon Tamponade in Cesarean Section for Placenta Previa Patients. PLoS One. 2015;10(8):e0134282.
-
Wagaarachchi PT, Graham WJ, Penney GC, McCaw-Binns A, Yeboah Antwi K, Hall MH. Holding up a mirror: changing obstetric practice through criterion-based clinical audit in developing countries. Int J Gynaecol Obstet. 2001;74(2):119–30; discussion 31. Epub 2001/08/15.
-
Maier RC. Control of postpartum haemorrhage with uterine packing. Am J Obstet Gynecol. 1993;169(2 Pt 1):317–332.
-
Bakri YN, Amri A, Abdul Jabbar F. Tamponade-balloon for obstetrical bleeding. Int J Gynaecol Obstet. 2001;74(2):139-142.
-
Vitthala S, Tsoumpou I, Anjum ZK, Aziz NA. Use of Bakri balloon in post-partum haemorrhage: a series of 15 cases. Aust N Z J Obstet Gynaecol. 2009;49(2):191-194.
-
Suarez S, Conde-Agudelo A, Borovac-Pinheiro A, et al. Uterine balloon tamponade for the treatment of postpartum hemorrhage: a systematic review and metaanalysis. Am J Obstet Gynecol. 2020;222(4):293.e1-293.e52.
-
Likis FE, Sathe NA, Morgans AK, et al. Management of Postpartum Hemorrhage. Rockville (MD): Agency for Healthcare Research and Quality (US); April 2015.
-
Ochoa M, Allaire AD, Stietly ML. Pyometra after hemostatic square suture technique. Obstet Gynecol. 2002;99(3):506–509.
-
Joshi VM, Shrivastava M. Necrosis of the uterus following a uterine brace compression suture. Br J Obstet Gynaecol. 2004;111(3):279–280.
-
Melamed N, Ben-Haroush A, Chen R, et al. Intrapartum cervical lacerations: characteristics, risk factors, and effects on subsequent pregnancies. Am J Obstet Gynecol. 2009;200(4):388.e1-4.
-
Belfort M, Kleinman S. Postpartum hemorrhage: Medical and minimally invasive management. UpToDate. 2019.
-
Lousquy R, Morel O, Soyer P, et al. Routine use of abdominopelvic ultrasonography in severe postpartum hemorrhage: retrospective evaluation in 125 patients. Am J Obstet Gynecol. 2011;204(3):232.e1-6.
-
Dali SM, Rajbhandari S, Shrestha S. Puerperal inversion of the uterus in Nepal: case reports and review of literature. J Obstet Gynaecol Res. 1997;23(3):19-25.
-
Baskett TF. Acute uterine inversion: a review of 40 cases. J Obstet Gynaecol Can. 2002;24(12):953-6.
-
Rudloff U, Joels LA, Marshall N. Inversion of the uterus at caesarean section. Arch Gynecol Obstet. 2004;269(3):224-6.
-
Huntington JL, Irving FC, Kellogg FS. Abdominal reposition in acute inversion of the puerperal uterus. Am J Obstet Gynecol. 1928;15(1):34-40.
-
Witteveen T, van Stralen G, Zwart J, van Roosmalen J. Puerperal uterine inversion in the Netherlands: a nationwide cohort study. Acta Obstet Gynecol Scand. 2013;92(3):334-7.
-
You WB, Zahn CM. Postpartum hemorrhage: abnormally adherent placenta, uterine inversion, and puerperal hematomas. Clin Obstet Gynecol. 2006;49(1):184-97.
-
Watson P, Besch N, Bowes WA Jr. Management of acute and subacute puerperal inversion of the uterus. Obstet Gynecol. 1980;55(1):12-16.
-
Kitchin JD 3rd, Thiagarajah S, May HV Jr, Thornton WN Jr. Puerperal inversion of the uterus. Am J Obstet Gynecol. 1975;123(1):51-8.
-
Platt LD, Druzin ML. Acute puerperal inversion of the uterus. Am J Obstet Gynecol. 1981;141(2):187-90.
-
Çetin BA, Aydogan Mathyk B, Atis Aydin A, et al. Comparing success rates of the Hayman compression suture and the Bakri balloon tamponade. J Matern Fetal Neonatal Med. 2019;32(18):3034–3038.
-
Kayem G, Kurinczuk JJ, Alfirevic Z, et al. Uterine compression sutures for the management of severe postpartum hemorrhage. Obstet Gynecol. 2011;117:14–20.
-
B-Lynch C, Coker A, lawai AH, Abu J, Cohen MJ. The B-Lynch surgical technique for the control of massive postpartum haemorrhage: an alternative to hysterectomy? Five cases reported. Br J Obstet Gynaecol. 1997;104(3):372-375.
-
James AH, Paglia MJ, Gernsheimer T, Grotegut C, Thames B. Blood component therapy in postpartum hemorrhage. Transfusion. 2009;49(11):2430–2433.
-
Grotegut C, Ahmadzia H, Peterson-Layne C, Lockhart E, James A. Management of Coagulopathy in Postpartum Hemorrhage. Seminars in Thrombosis and Hemostasis. 2016;42(07):724–731.