O actualizare a ghidurilor internaţionale privind screeningul cancerului de sân
Update of international guidelines for breast cancer screening
Abstract
In the last decade, the number of breast cancer cases has increased by 35% worldwide. The screening mammography clearly reduces the relative risk of breast cancer mortality in women between 50 and 59 years old, the decrease being more important for the age range 60-69 years old. The United States of America together with international specialized groups recommend mammographic screening in women between 50 and 74 years old every 1-2 years, depending on the individual risk factors and on the patient’s desire. Japan’s position is that of recommending mammographic screening without clinical examination of the breasts for women between the ages of 40 and 74. In the European Union, population screening by mammography in women aged 50-69 has been recognized as a priority, along with the screening for cervical and colon cancer, since 2003. Romania presents a major delay in the implementation of organized screening of the population for breast cancer compared to other EU members, although the number of cases is increasing.Keywords
breast cancerscreening mammographyBRCA 1/2PTEN or TP53 genesmolecular genetic testsRezumat
În ultimul deceniu, numărul cazurilor de cancer de sân a crescut cu 35% în întreaga lume. Screeningul prin mamografie determină scăderea certă a riscului relativ de mortalitate prin cancer de sân la femeile între 50 şi 59 de ani, reducerea fiind mai importantă pentru intervalul de vârstă 60-69 de ani. Statele Unite ale Americii, în acord cu grupurile internaţionale de specialitate, recomandă screeningul mamografic al femeilor între 50 şi 74 de ani la fiecare 1-2 ani, în funcţie de factorii individuali de risc şi de dorinţa pacientei. Poziţia Japoniei constă în recomandarea screeningului mamografic fără examinarea clinică a sânilor pentru femeile cu vârsta cuprinsă între 40 si 74 de ani. În Uniunea Europeană, screeningul populaţional prin mamografie la femeile cu vârste cuprinse între 50 şi 69 de ani a fost recunoscut drept o prioritate, alături de screeningul pentru cancerul de col uterin şi de colon, încă din 2003. România înregistrează o întârziere majoră în implementarea screeningului organizat al populaţiei pentru cancerul de sân, comparativ cu alte state ale UE, deşi numărul de cazuri este în continuă creştere.Cuvinte Cheie
cancer mamarscreening mamograficgene BRCA 1/2PTENTP53teste de genetică molecularăBreast cancer is the leading cause of death from non-melanoma skin cancer in women worldwide. In the United States of America, most breast cancers are diagnosed after the screening for suspected abnormalities and this approach reduces both cancer mortality and facilitates early diagnosis, which has led to a dramatically reduced breast cancer mortality along with improved treatment methods after the 1980s(1,2).
In the last decade (2005-2015), the number of breast cancer cases has increased by 35% worldwide and about one-fifth of this increase is due to increased incidence rates(3). The incidence of breast cancer (age-standardized rate) decreased only in countries with high social development indicator (SDI), all other countries presenting an increase of the indicator(3). Regarding mortality, the number of deaths worldwide increased by 21%, but the standardized death rate according to age decreased globally by 6%, a decrease in mortality being observed especially in countries with high and medium SDI(4).
The lifetime risk of developing breast cancer includes three categories: medium risk (less than 15%), moderate risk (approximately 15-20%) and high risk (more than 20%). The main factors used in determining the risk category include: personal history of breast, ovarian, tubal or peritoneal cancer; family history of breast, ovarian, tubal or peritoneal cancer; ethnicity (e.g., Ashkenazi Jewish population) associated with BRCA 1 or 2 mutations, known carriers pathogenic mutations involved in hereditary syndromic breast and ovarian cancer and their relatives, breasts with high mammographic density, high-risk lesions detected by prior breast biopsy (atypical hyperplasia), age of onset of menstruation, age of first birth, number of pregnancies and menopausal status, as well as chest radiotherapy performed at the age of 10-30 years old. Women who do not have any of these risk factors, representing the vast majority, are considered to belong to the average lifetime risk of developing breast cancer, estimated at 12.4%(5). Many predictive models try to stratify the population as accurately as possible depending on the share of these risk factors, trying to assess the risk of pathogenic mutations involving BRCA 1/2, PTEN or TP53 genes, based on personal and family history.
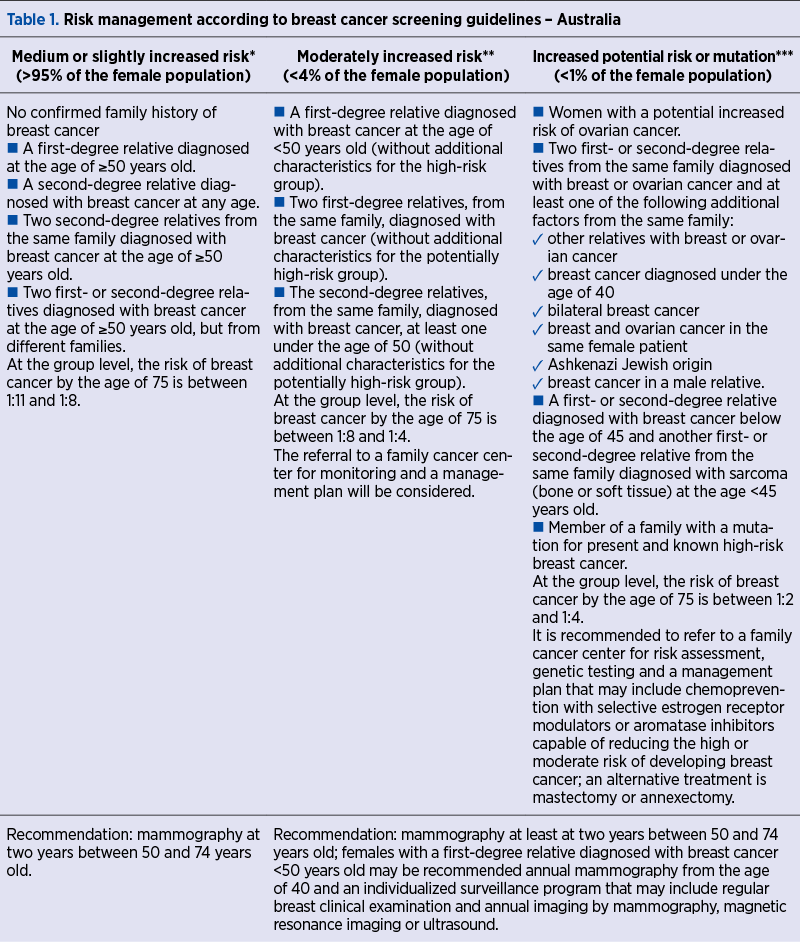
The Australian Guideline model sets out a specific screening management according to the risk group (Table 1).
The carcinogenetic effects of BRCA 1 and 2, PTEN and TP53 gene mutations are extensively discussed in the subchapter entitled “Tests of molecular genetics and immunohistochemistry” of the monograph Diagnosis of Endometrial Pathology(6).
Comprehensive genotyping through next-generation sequencing techniques will become economically feasible soon, classifying tumors based on several essential and recurring molecular events, finding both clinical and research applicability.
The conclusion is that patients with molecular tumor stages and/or family history suggestive for any form of genetic cancer should be referred to a specialist in medical genetics for counseling at a center specializing in assessing the increased risk of cancer that both them and their families have. Although the medical genetics specialty has been included for many years in the nomenclature of specialties of the Ministry of Health, the role of these doctors in the medical practice in Romania is still marginalized, in the context of the absence of specialized centers of medical genetics and the lack of functionality of the National Cancer and Rare Diseases Registries.
The family history of cancer explored over three generations on all continents of the world, and illustrated in Table 1, can only be correctly performed by a specialist in the field, able to subsequently indicate the necessary molecular genetic tests and monitor the risk of family aggregates through a standardized functional database.
For the medium-risk category, the most widely represented, age is the most important factor driving the screening program, given that the incidence of breast cancer increases with age, being low below 40 years old and increasing progressively beyond this threshold. The sensitivity and the specificity of mammography are also age-dependent, being higher in the elderly compared to young women(7), the exploration detecting 73% of breast cancers in women in the fifth decade of life(8), compared to 85% of breast cancers detected in women over 60 years old. Under the age of 40, there is no standard routine recommendation for most women in the medium-risk group, the positive predictive value for mammography between the ages of 35 and 39 being only 1.3%(9).
For the 40-49 years old age group, most US experts encourage screening mammography based on informed patient consent after discussing the advantages and disadvantages of the examination at this age, given that European guidelines recommend the initiation of screening at the age of 50. For women who decide to initiate the screening from the age of 40 years old, US specialists suggest having a mammogram every 1-2 years. The benefit of screening after 40 years old is relatively uncertain. Considering the number of potential life years saved by screening in the fifth decade of life, the effort seems justified, but the number of cases prevented by mortality due to breast cancer is relatively low. A systematic review and meta-analysis of nine randomized trials shows that, for every 10,000 women scanned over ten years, three deaths from breast cancer are prevented(10).
Similarly, the Age Trial performed in the UK states that breast cancer mortality analyzed over a 10.7-year period was lower in the group of women who initiated screening mammography at the age of 40 compared to the standard screening age, but the difference is not statistically significant(11). Given the modest benefit of screening between 40 and 49 years old, the disadvantages seem to be more consistent, the false-positive results being more common in this age group. Speaking about the disadvantages of screening mammography, three major elements are considered: radiation exposure, false-positive cases and overdiagnosis.
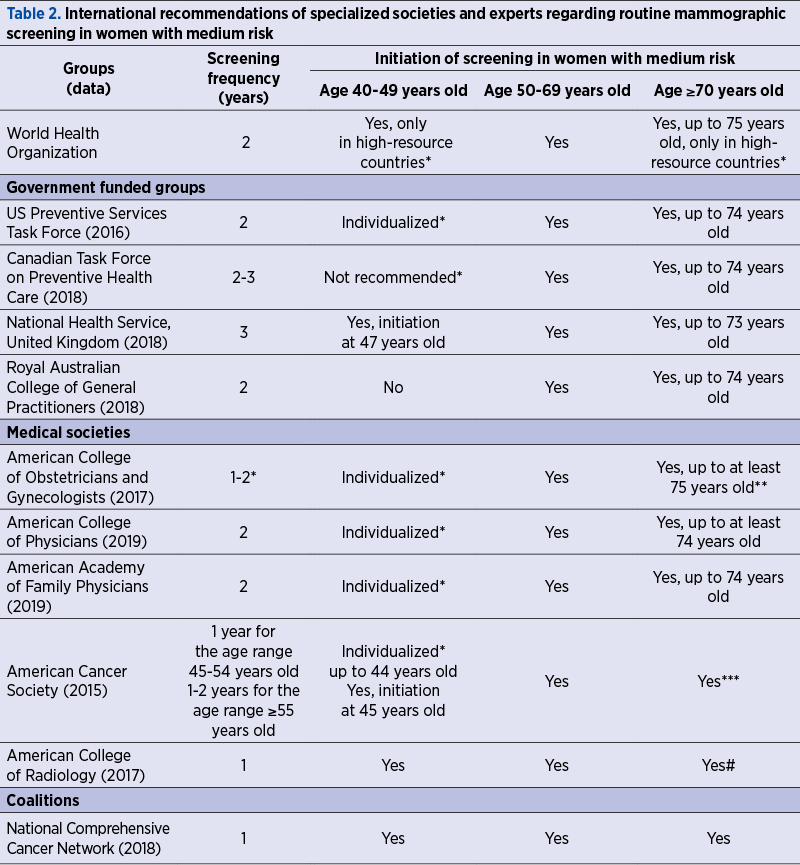
The United States together with the international specialized groups (Table 2) recommend mammographic screening between 50 and 74 years old, every 1-2 years, depending on the individual risk factors and the patient’s desire(12-23).
The systematic review published in 2016 on breast screening by mammography states a definite decrease in the relative risk of breast cancer mortality in women between 50 and 59 years old, the reduction being more important for the age range 60-69 years old(10). Screening also reduces the risk of advanced breast cancer in women over the age of 50, which supports the definite benefit of applying this method.
Most expert groups recommend routine screening mammography for women between the ages of 50 and 74, the frequency of investigation being variable in this age group. For women over 75 years old, screening will only be offered if their life expectancy is at least 10 years, with the investigation being recommended every two years(17). Given that the ideal upper limit and the frequency of screening are not clearly established, the indication should consider that the incidence of breast cancer remains high after the age of 80, but the number of years saved decreases with age. Mammographic screening of older women can lead to early diagnosis, but it does not decrease mortality; this statement is supported by the following aspects: the lower the life expectancy, the lower the potential of screening to prolong life; the incidence of ductal carcinoma in situ (DCIS) increases with age, but it is not yet clear to what extent the treatment of this pathology decreases mortality.
The International Agency for Research on Cancer states that data showing a reduction in breast cancer mortality in women aged 40-44 years old through mammographic screening are limited, but data showing that mammographic screening reduces breast cancer mortality and the benefit substantially outweighs the risk of radiation are enough to support the routine screening of all women between the ages of 50 and 75 years old. Regarding the clinical examination, the Agency states that there is not enough data to show that the clinical examination of the breast reduces the mortality from breast cancer, but the data suggest that, using the clinical examination, the tumors detected would be at an earlier stage.
The position of the Japanese National Cancer Center (2016) is as follows: for women aged 40 to 74, mammographic screening without clinical examination of the breasts is recommended; for women aged 40-64, both population and opportunistic screening, based on mammography associated with clinical examination of the breast, have been shown to statistically significantly reduce mortality from breast cancer, while breast ultrasound and clinical examination are not recommended as single screening methods for breast cancer(23).
The American Cancer Society (ACS) recommends the initiation of mammographic screening at the age of 45 years old, given that the absolute risk of breast cancer occurrence and mortality in women aged 45 to 49 is almost similar to that in women between the ages of 50 and 54 years old. ACS recommends continuing the annual screening until the age of 55, after this age the investigation being recommended every two years.
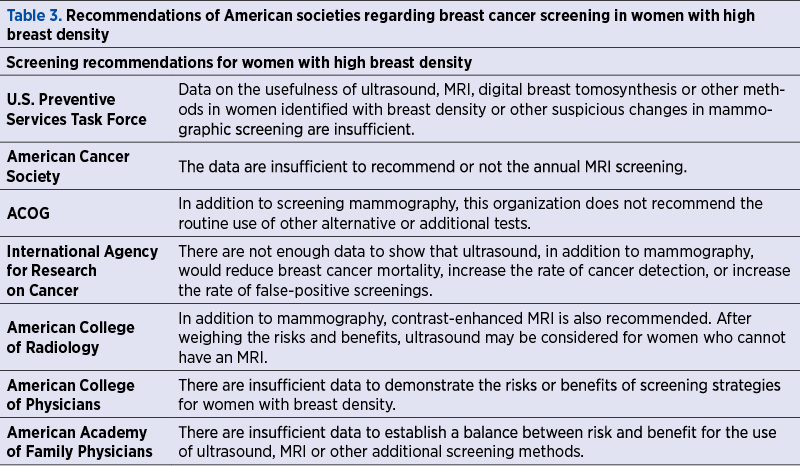
Randomized trials have shown that mammographic screening at two years certainly reduces breast cancer mortality. A systematic review of the 2016 US Preventive Services Task Force, representing an update of breast cancer screening recommendations, states more favorable biopsy rates and the cumulative risk of false positives associated with biennial screening compared to annual screening. The optimal interval between mammographic examinations continues to remain variable, age, breast density and the use of hormonal therapy being the most important factors in modeling the exploratory frequency in screening programs. The recommendations of the American Societies regarding the exploration of breast density are presented in Table 3, the consensus being obvious.
Film, digital or tomosynthesis (3D) mammography represent the main methods of screening the medium-risk female population. Other imaging techniques, including ultrasonography and MRI, are reserved for subsequent evaluations of mammograms or for the screening of women at high risk for breast cancer, although their benefit as adjuvant methods for mammography remains controversial.
The indication of the clinical breast examination is limited to the evaluation of women with breast symptoms or abnormalities detected by self-examination, not being any evidence of the benefit of using the clinical examination with or without mammography in improving the patient’s prognosis, but only in increasing the rate of false-positive results. Despite the lack of proven efficacy, the recommendations of major companies in the field differ, World Health Organization (WHO) considering clinical examination as an appropriate method for women aged 50 to 69 from countries with limited resources or poor health systems, where screening mammography at recommended standards could not be organized. Several studies consider self-examination to be of no benefit in the context of an increase in the rate of benign breast biopsy. Many groups of experts encourage the education of women in order to differentiate normal breast tissue from various suspicious formations, but especially in order to refer to medical services in case of detecting any breast abnormalities, WHO assigning to this technique the role of awareness of potential risk by women without any recognition of the screening valences of the method.
However, mammography can lose 20% of breast cancers screening(24). The choice of the type of mammographic examination generally depends on availability. The most used technique in Europe and the US is digital mammography, which is about to be quickly replaced by breast tomosynthesis. For women with breast density, digital mammography or tomosynthesis, techniques with a higher sensitivity, are preferred. The sensitivity of mammography – especially of the film mammography – is inversely correlated with breast density. Regarding mammography technique, women should be informed about the transient aspect of breast compression, which is important to reduce movement artifacts, to improve image quality and to reduce the amount of irradiation required for the investigation. A comparative analysis of previous mammograms is beneficial to current mammographic interpretation. Mammography is not routinely indicated, but postmastectomy for malignancy.
Screening ultrasonography is not recommended for the medium-risk group, as it is not evaluated as a screening strategy designed to reduce breast cancer mortality, even in breast density (Table 3). In some states of the USA, ultrasonography is mentioned as a complementary method to mammography in patients with high breast density, often being used to monitor mammographically detected lesions in order to establish their evolutionary potential.
MRI screening is also not recommended for medium-risk patients, according to the practice guide issued by ACS. Combined with mammography, magnetic resonance imaging is used in the primary screening of high-risk patients. Patients in the moderate risk group (15-20% risk of developing breast cancer during life) seem to benefit from the complementarity of ultrasound and MRI, supplementing the basic mammographic screening, but none of the modalities is usually covered by health insurance in most countries of the world. Women in the high-risk group have a clear benefit in corroborating imaging techniques and in increasing the frequency of examination.
Women with breast augmentation by implant insertion benefit from the same indications and frequency of screening, with the mention of the increased risk of omission of small lesions due to the radiopacity of implants and the recommendation to use four planes per breast.
Breast biopsies are performed for 1-2% of mammographic examinations and have the effect of decreasing the specificity and positive predictive value of the screening performed after the biopsy. The European Guidelines for Quality Assurance in Breast Cancer Screening(25) updates the indications and technique for vacuum-assisted needle core biopsy (VANCB) that provides a considerable volume of tissue for histopathological analysis of microcalcifications, rapidly evacuating the hematoma from the biopsy site.
The most recommended techniques for investigating breasts during pregnancy or lactation are digital mammography and tomosynthesis; the use of the apron and the protection of the fetus from the effects of irradiation in the first eight weeks of life, with maximum susceptibility, are measures considered sufficient enough to be able to use mammography in pregnancy, in individualized cases, in which it is considered absolutely necessary (the use of apron exposes the fetus to irradiation below 0.03 mGy). Palpable lesions in pregnancy are approached by first-line ultrasound, mammography being indicated only for ultrasound lesions suspected of malignancy.
Postmenopausal hormone replacement therapy inhibits the involution of the breast tissue, whose density increases, which decreases the sensitivity of mammographic examination.
Axillary lymphadenopathy, rarely reported after the administration of BCG, influenza or HPV vaccines, has an increased incidence rate after the administration of messenger RNA-based COVID-19 vaccines, therefore the Society of Breast Imaging recommends planning mammographic screening before the first dose of anti-COVID-19 mRNA vaccines or at 4-6 weeks from the second dose(26).
The benefits of screening mammography are mainly represented by a decrease in breast cancer mortality by about 20%, without this reduction to be certainly attributed to advances in imaging or to improved therapeutic efficacy(27-29). Also, the contribution of mammographic screening to reducing mortality varies significantly between the different molecular subtypes of this neoplasia.
The disadvantages associated with breast cancer screening include the possible false-positive lesions, anxiety, overdiagnosis and unnecessary treatment associated with its risks. The overdiagnosis of breast cancer represented by the identification of neoplastic breast lesions unable to determine clinical consequences during a woman’s lifetime is estimated at 10% to 50% of all women diagnosed with breast cancer(30); the variability of this assessment is found in relation to the differences in the definition of breast cancer (ductal carcinoma in situ may or may not be included in the study group, the age of the study group being also important). There are studies which suggest that many types of cancer, especially those limited to the mammary ducts, are biologically insignificant and could never become clinically evident during the patient’s lifetime. Given the fact that it is impossible to distinguish between biologically insignificant and those capable of increasing, metastasizing and generating death, the idea of consecutive overdiagnosis and overtreatment should be seen as an indication of individualized approach to screening results, which should not diminish the adherence of the medical staff and of the patients to the screening program. Factors that increase the rate of false-positive mammograms are young age, increased breast density, family history of breast cancer, previous breast biopsies, estrogen use, three-year interval between screenings, lack of previous comparative imaging, and individual tendency of overdiagnosis of the radiologist. The false positive rate of screening mammography in the US is 10%, but rises to 50% at 10 years of annual screening; this rate is much lower in other states(31). Although irradiation may increase the risk of breast cancer, the dose of radiation administered by mammographic screening is low enough that the benefit of screening in women over 40 years old exceeds the risk of irradiation by mammography. However, this risk of irradiation becomes significant in BRCA 1 and BRCA 2 mutation carriers.
In the European Union, population screening by mammography in women aged 50-69 has been recognized as a priority, along with screening for cervical and colon cancer, since 2003(32). The implementation of this recommendation has been repeatedly assessed: the 2008 report revealed the existence of population-based cancer screening programs in only 11 countries(33); the second evaluation found significant progress in improving access to breast cancer screening, with 79% of the eligible women being covered by the screening invitation and 49% being covered by examination(34). However, there are still large variations in the EU in terms of access to breast cancer screening, as well as the incidence and mortality of this disease. In general, women in socioeconomically disadvantaged areas have lower access to screening, lower incidence of breast cancer, but higher mortality rates(34,35).
Romania presents a major delay in implementing organized screening of the population for breast cancer, compared to other EU members. In the year of EU joining (2007), breast cancer screening was being planned(33). In 2017, the foundations of a small-scale screening pilot project were laid(34). Attempts to provide free access to mammographic screening for women of eligible age – either as a service included in the package of basic health services, or in projects – have had a very low impact, 79% of the women in Romania declaring that they never had an X-ray of the breasts(36,37).
In Romania, breast cancer has registered an increase in the number of cases over the years and represents a constant proportion of 16% of all deaths caused by cancer. The mortality model is worsening and this is widening the health gap compared to the female population in the EU. All the data obtained and published by us(36) represent urgent reasons for planning and implementing an organized program of population screening for breast cancer as quickly and efficiently as possible.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
-
Plevritis SK, Munoz D, Kurian AW, Stout NK, Alagoz O, Near AM, Lee SJ, van den Broek JJ, Huang X, Schechter CB, Sprague BL, Song J, de Koning HJ, et al. Association of screening and treatment with breast cancer mortality by molecular subtype in US women, 2000-2012. JAMA. 2018;319(2):154.
-
Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367(21):1998-2005.
-
Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, Dandona L, et al. Global burden of disease cancer collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3(4):524-48.
-
Vos T, et al. GBD 2015: Mortality and causes of deaths collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545-1602.
-
Arbyn M, Anttila A, Jordan J, Schenck U, Ronco G, Segnan N, Wiener H, Herbert A, Daniel J, von Karsa L (eds.). European guidelines for quality assurance in cervical cancer screening. 2th Ed. European Commission, Office for Official Publications of the European Communities, Luxembourg. 2008. Available at: http://bookshop.europa.eu/is-bin/INTERSHOP.enfinity/WFS/EU-Bookshop-Site/en_GB/- /EUR/ViewPublication-Start?PublicationKey=ND7007117.
-
Bohîlţea RE. Diagnosticul patologiei endometriale. Ed. Univ. „Carol Davila” Bucureşti, 2018.
-
Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L; U.S. Preventive Services Task Force. Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151(10):727-37.
-
NCI-funded Breast Cancer Surveillance Consortium (HHSN26121100031C). http://breastscreening.cancer.gov/.
-
Yankaskas BC, Haneuse S, Kapp JM, Kerlikowske K, Geller B, Buist DS. Breast Cancer Surveillance Consortium. Performance of first mammography examination in women younger than 40 years. J Natl Cancer Inst. 2010;102(10):692-701.
-
Nelson HD, Fu R, Cantor A, Pappas M, Daeges M, Humphrey L. Effectiveness of breast cancer screening: Systematic review and meta-analysis to update the 2009 U.S. Preventive Services Task Force Recommendation. Ann Intern Med. 2016;164(4):244-55.
-
Moss SM, Cuckle H, Evans A, Johns L, Waller M, Bobrow L. Age Trial Management Group. Effect of mammographic screening from age 40 years on breast cancer mortality at 10 years’ follow-up: a randomised controlled trial. Lancet. 2006;368(9552):2053-60.
-
WHO position paper on mammography screening. WHO, Geneva, 2014.
-
US Preventive Services Task Force. Screening for Breast Cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279-96.
-
Klarenbach S, Sims-Jones N, Lewin G, et al. Recommendations on screening for breast cancer in women aged 40-74 years who are not at increased risk for breast cancer. CMAJ. 2018;90(49):E1441-E1451.
-
National Health Service. When it’s offered: Breast cancer screening. Available at: https://www.nhs.uk/conditions/breast-cancer-screening/when-its-offered/ (Accessed on June 18, 2019).
-
The Royal Australian College of General Practitioners. Guidelines for preventive activities in general practice, 9th ed, East Melbourne, RACGP, 2018.
-
American College of Obstetricians-Gynecologists. Practice bulletin no. 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130(1):e1-e16.
-
Qaseem A, Lin JS, Mustafa RA, et al. Screening for breast cancer in average-risk women: A guidance statement from the American College of Physicians. Ann Intern Med. 2019;170(8):547-60.
-
American Academy of Family Physicians. Clinical preventive service recommendation: Breast cancer. Available at: www.aafp.org/patient-care/clinical-recommendations/all/breast-cancer.html (Accessed on June 27, 2019).
-
Oeffinger KC, Fontham ETH, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599-614.
-
Mainiero MB, Lourenco A, Mahoney MC, et al. ACR Appropriateness Criteria Breast Cancer Screening. J Am Coll Radiol. 2013;10(1):11-4.
-
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in oncology: Breast cancer, v2, 2018.
-
Japanese Research Group for the Development of Breast Cancer Screening Guidelines; Hamashima C, Hattori M, Honjo S, Kasahara Y, Katayama T, et al. Japanese Research Group for the Development of Breast Cancer Screening Guidelines. The Japanese Guidelines for Breast Cancer Screening. Jpn J Clin Oncol. 2016;46(5):482-92.
-
Breast Cancer Surveillance Consortium, funded by the National Cancer Institute. Available at: http://breastscreening.cancer.gov/.
-
Perry N, Broeders M, de Wolf C, Tornberg S, Holland R, von Karsa L. European guidelines for quality assurance in breast cancer screening and diagnosis. 4th Ed Suppl. European Commission, Office for Official Publications of the European Union, Luxembourg, 2013.
-
Helsper CW, Campbell C, Emery J, Neal RD, Li L, Rubin G, van Weert H, Vedsted P, Walter FM, Weller D, Nekhlyudov L. Cancer has not gone away: A primary care perspective to support a balanced approach for timely cancer diagnosis during COVID-19. Eur J Cancer Care (Engl). 2020;29(5):e13290.
-
Miller AB, Wall C, Baines CJ, Sun P, To T, Narod SA. Twenty five year follow-up for breast cancer incidence and mortality of the Canadian National Breast Screening Study: randomised screening trial. BMJ. 2014;348:g366.
-
Bjurstam N, Björneld L, Warwick J, Sala E, Duffy SW, Nyström L, Walker N, Cahlin E, Eriksson O, Hafström LO, Lingaas H, Mattsson J, Persson S, Rudenstam CM, Salander H, Säve-Söderbergh J, Wahlin T. The Gothenburg Breast Screening Trial. Cancer. 2003;97(10):2387-96.
-
Andersson I, Janzon L. Reduced breast cancer mortality in women under age 50: updated results from the Malmö Mammographic Screening Program. J Natl Cancer Inst Monogr. 1997;22:63-7.
-
Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: an independent review. Lancet. 2012 Nov;380(9855):1778-86.
-
Jørgensen KJ, Gøtzsche PC, Kalager M, Zahl PH. Breast Cancer Screening in Denmark: A Cohort Study of Tumor Size and Overdiagnosis. Ann Intern Med. 2017;166(5):313-23.
-
European Council. Council recommendation of 2 December 2003 on cancer screening (2003/878/EC). Off J Eur Union. 2003;327:34–8.
-
Von Karsa L, Anttila A, Ronco G, von Karsa L, Ponti A, Malila N, Arbyn M, Segnan N, Castillo-Beltran M, Boniol M, Ferlay J, et al. Cancer screening in the European Union. Report on the implementation of the Council Recommendation on cancer screening. First report. Brussels: European Commission; 2008.
-
Ponti A, Anttila A, Ronco G, Senore C. Cancer Screening in the European Union. Report on the implementation of Council Recommendation on Cancer Screening. Brussels: European Commission, 2017 (Accesed on 21 March, 2021).
-
Deandrea S, Molina-Barceló A, Uluturk A, Moreno J, Neamtiu L, Peiró-Pérez R, Saz-Parkinson Z, Lopez-Alcalde J, Lerda D, Salas D. Presence, characteristics and equity of access to breast cancer screening programmes in 27 European countries in 2010 and 2014. Results from an international survey. Prev Med. 2016;91:250-63.
-
Furtunescu F, Bohîlţea RE, Voinea S, Neacşu A, Pop CS. Breast cancer mortality gaps in Romanian women compared to EU after ten years of accession – Is breast cancer screening a priority for action in Romania?. Exp Therc Med. 2021;21:268-73.
-
Smith D, Thomson K, Bambra C, Todd A. The breast cancer paradox: A systematic review of the association between area-level deprivation and breast cancer screening uptake in Europe. Cancer Epidemiol. 2019;60:77-85.



