Şocul anafilactic este o reacţie severă, acută, generalizată, de hipersensibilitate, care apare rar în timpul sarcinii, dar care are efecte adverse semnificative pentru femeia însărcinată şi făt, din cauza hipotensiunii. Prezentăm o minirevizuire privind diagnosticul clinic şi managementul sarcinii, pe baza cazurilor descrise în literatură. În timp ce etiologia sa este diversă, cele mai frecvente cazuri de şoc anafilactic sunt reprezentate de administrarea de antibiotice, în special cele pentru profilaxia infecţiei streptococice de grup B, fiind urmate, în ordinea frecvenţei, de medicamentele anestezice. Consecinţele materne ale şocului anafilactic sunt diverse, pe lângă hipotensiune, manifestările sale fiind cutanate, digestive, cardiovasculare sau respiratorii. Implicaţiile fetale pot include bradicardie fetală, encefalopatie ischemică hipoxică sau chiar moarte fetală. Adrenalina injectată intramuscular în vastul lateral al cvadricepsului (coapsa anterolaterală) este tratamentul de primă linie al anafilaxiei, prin urmare ar trebui să fie disponibilă rapid pentru femeile gravide cu risc potenţial de anafilaxie. Triptaza serică este singurul biomarker validat disponibil pentru anafilaxie.
Şocul anafilactic în sarcină: minireview
Anaphylactic shock in pregnancy: a mini-review
First published: 28 octombrie 2021
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/ObsGin.69.3.2021.5555
Abstract
Rezumat
Anaphylactic shock is a severe, acute, generalized, hypersensitivity reaction that appears rarely during pregnancy, but which has significant adverse outcomes on the pregnant woman and the fetus due to hypotension. Herein we present a mini-review about the clinical diagnosis and management in pregnancy, based on cases described in literature. While its etiology is diverse, the most common cases of anaphylactic shock are represented by the administration of antibiotics, especially those for the prophylaxis of group B streptococcal infection, being followed, in the frequency order, by the anesthetic drugs. The maternal consequences of anaphylactic shock are various, besides hypotension, its manifestations being cutaneous, digestive, cardiovascular or respiratory. The fetal implications may include fetal bradycardia, ischemic hypoxic encephalopathy or even death. Adrenaline intramuscularly injected into the vastus lateralis of the quadriceps (anterolateral thigh) is the first-line treatment of anaphylaxis, therefore it should be rapidly available for pregnant women with potential risk of anaphylaxis. Serum tryptase is the only available validated biomarker for anaphylaxis.
Introduction
Anaphylactic shock is a severe, acute, generalized, hypersensitivity reaction that appears rarely during pregnancy, but which has significant adverse outcomes on the pregnant woman and the fetus due to hypotension. While its etiology is diverse, the most common cases of anaphylactic shock are represented by the administration of antibiotics(1,2), especially the ones for the prophylaxis of group B streptococcal infection(2-4), being followed, in the frequency order, by the anesthetic drugs. Anaphylactic shock is rare during pregnancy, with a reported prevalence of 2.7 cases per 100,000 births in the United States in 2010(2) and an incidence of 3.8 per 100,000 pregnant women hospitalized in 2019(5).
The maternal consequences of anaphylactic shock are various, besides hypotension, its manifestations being cutaneous, digestive, cardiovascular or related to the respiratory system. Adrenaline intramuscularly injected into the vastus lateralis of the quadriceps (anterolateral thigh) is the first-line treatment of anaphylaxis, therefore it should be rapidly available for pregnant women with a potential risk of anaphylaxis. Serum tryptase is the only available validated biomarker for anaphylaxis.
Anaphylaxis is a systemic reaction mediated by many mediators, including vasoactive substances (amines), released mainly by mast cells and basophils. Their release is often triggered by specific IgE antibodies, with rapid onset of clinical manifestations, involving at least two of the following systems: cutaneous, cardiovascular, respiratory or gastrointestinal(6). New studies have also shown that painful uterine contractions may be a manifestation of anaphylaxis, in both pregnant and nonpregnant women, but this is not frequently observed(7).
In pregnant women, the symptoms for anaphylactic shock may also involve, besides hypotension, low back pain, vulvar and vaginal itching, fetal distress or premature birth(8). The fetal implications may include fetal bradycardia, ischemic hypoxic encephalopathy or even death.
Herein we present a mini-review about the clinical diagnosis and management in pregnancy, based on cases described in literature.
Etiology
The etiology is very diverse, but the most frequent triggers for anaphylactic shock are: antibiotics, muscle relaxants(9), nonsteroidal anti-inflammatory drugs, contrast agents, iron(10,11), venom or antivenom(12), food or latex(13-16).
In this regard, there is an European study conducted in 2020(5) which captured the proportion of each etiological agents in a group of 63 pregnant women or after birth who have suffered an anaphylactic shock.
Out of the 63 cases, 35 episodes of anaphylactic shock occurred before birth, while the remaining 28 occurred after birth, as follows:
n
16 patients with antepartum reactions caused by:
antibiotics (six cases)
iron i.v. (three cases)
influenza and pertussis vaccine (two cases)
food (two cases)
anesthesia (one case)
spasmolytics (one case)
omeprazole (one case).
n
19 patients with reactions immediately before birth caused by:
antibiotic prophylaxis preceding caesarean section (eight cases)
antibiotic prophylaxis for group B streptococcus (three cases)
prophylactic antibiotic therapy administered in case of premature rupture of membranes (two cases)
anesthesia (four cases)
oxytocin (one case)
food (one case).
n
Out of the 28 cases of anaphylactic shock after birth, 14 occurred after caesarean section and 14 occurred after vaginal birth, and were caused by:
antibiotic prophylaxis administered after caesarean section (seven cases)
anesthesia (three cases)
oxytocin (two cases)
gelofusine (two cases)
blood products (four cases).
Ergomet® (one case)
prostaglandins (one case)
antibiotic administered postpartum vaginally (one case)
carboprost (one case)
anesthetic agents used in natural birth (three cases)
gelofusin (one case)
nonsteroidal anti-inflammatory drugs (one case)
idiopathic (one case).
The first recorded case of anaphylaxis in a pregnant woman was in 1974, determined by the administration of succinylcholine(17). There are also cases of progesterone-triggered anaphylaxis recorded in the literature. Taking into account the fact that progesterone levels increase up to 10 times higher during pregnancy, recurrent anaphylaxis during the second and third trimesters in a pregnant woman was described(18).
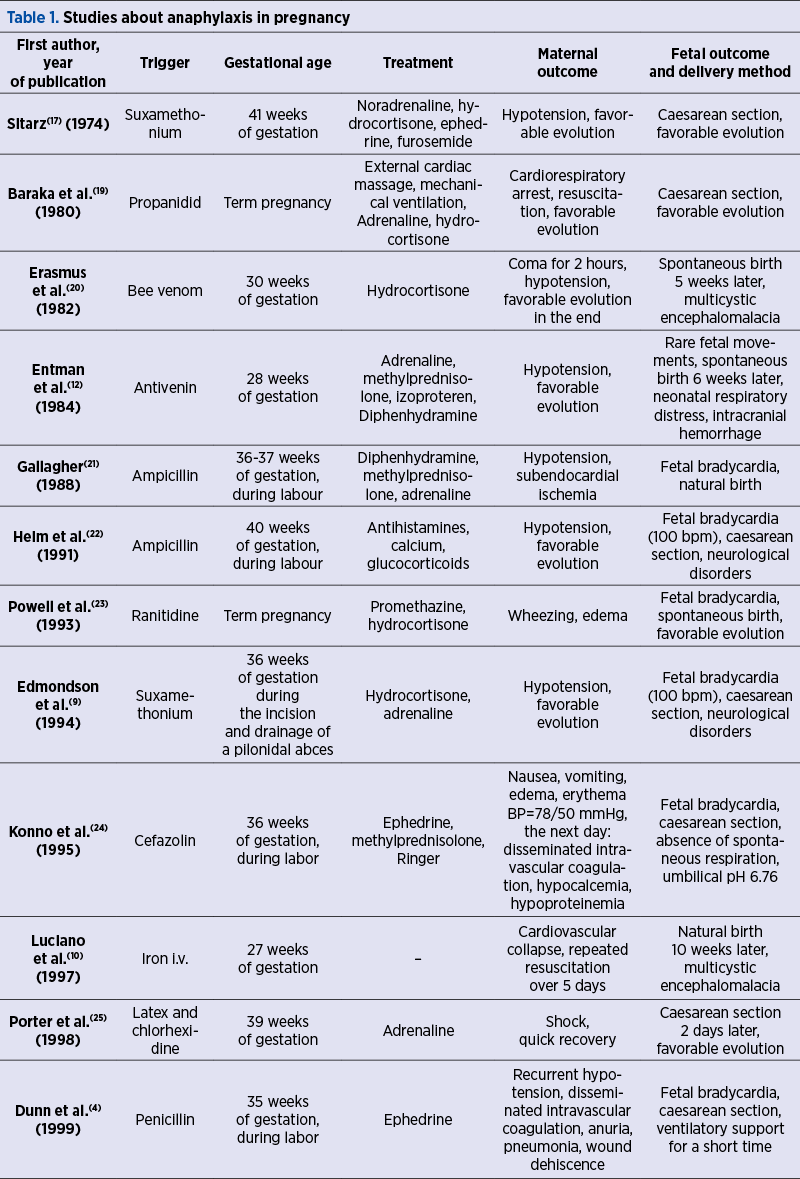
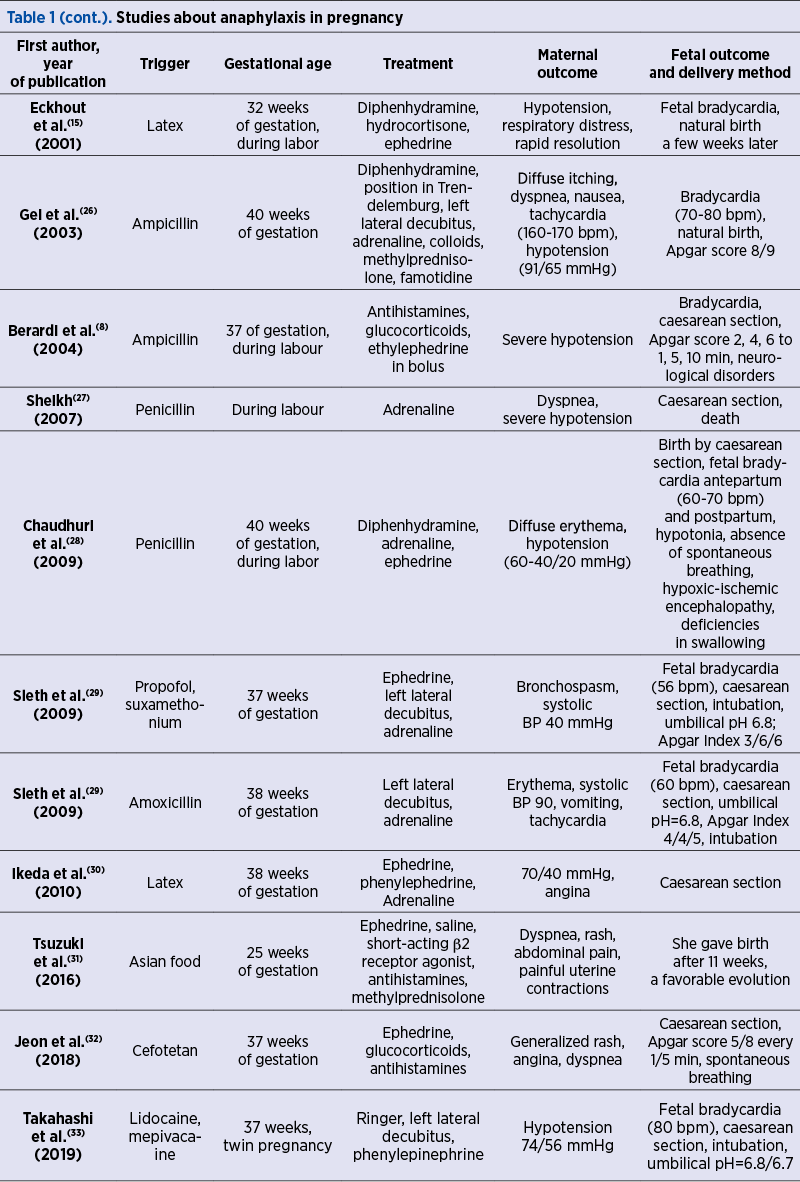
In Table 1, there are listed studies that are primary about the anaphylactic shock in pregnant women, from 1974 to 2019. The gestational age, the etiological agent, the maternal as well as the fetal evolution, and the corresponding treatment plan used are mentioned. The variety of etiopathogenic factors and the clinical manifestations of anaphylactic shock during pregnancy and peripartum emerge from this summary of existing studies. It also justifies the need for a treatment protocol, given the variety of attitudes towards this pathology.
Regarding neonatal complications, the hypoperfusion of the uteroplacental territory determines a fetal hypoxic status that manages to be counterbalanced for a short time by the redistribution of blood flow to the central territories. However, once this compensation system is overcome, the fetus shows signs of hypoxic-ischemic encephalopathy, leading in due course to numerous neurological consequences, with possible permanent damages to various brain structures(5,8,29,34).
When maternal cardiorespiratory arrest happens, emergency caesarean section is required(35,36). Although 90% of the newborns removed intraoperatively within 5 minutes of shock initiation show no signs of nervous system impairment, it is important that the fetus is removed as soon as possible in order to avoid the permanent impairment of the neurological functions. In the most severe cases, peripartum fetal hypoxic status can lead to death(33).
The differential diagnosis should be made with amniotic fluid embolism, sepsis triggered especially by group B streptococcal infection, vasovagal syncope, laryngeal stridor or cardiovascular collapse(37).
Pathophysiology
During pregnancy, the maternal immune system undergoes numerous changes in order to tolerate the product of conception. Because of this, cytokine signaling, suppression of the T cell response or alteration of cell-mediated immunity lead to a higher risk of pregnant women to have an abnormal immune response(38). Among other significant changes, there are also the increase in Th2-type cytokine production and the inhibition of cytokine production by Th1 cells(38-41).
IgE antibodies play the most important role in triggering an anaphylactic reaction. Physiologically, they are the weakest isotype represented in circulation (50-200 ng/ml of blood), and their concentration is significantly increased in people with atopy(42,43). IgE binds to the high-affinity receptor FceRI mainly on the surface of basophils and mast cells(44). At allergen exposure, the link between specific IgE and FceRI activates these cells and causes the immediate release of preformed mediators such as histamine, various proteases, and later on the release of de novo mediators such as leukotrienes, prostaglandins and cytokines(42,44).
Management of anaphylactic shock
in pregnant women
The treatment of anaphylaxis of pregnant patients in acute care settings is basically the same as in nonpregnant patients. The treatment of anaphylactic shock begins by stopping the trigger exposure, with patient’s assessment of airway, breathing, circulation, mental status, skin and body weight, and positioning the pregnant patient on her left side or in a position of comfort, while manual displacement of the gravid uterus to the left might be necessary. Intravenous access using two catheters with wide-bore cannula for fluid resuscitation by administering rapidly 0.9% (isotonic) saline is necessary, while large intravenous fluid volumes may be needed. Supplemental oxygen up to 100%, 6-8 l/minute, as needed, through a face mask or oropharyngeal airway is important. Fetal monitoring and maternal monitoring of blood pressure, cardiac rate and function, respiratory status and oxygenation are mandatory(6,45,46).
Adrenaline (epinephrine) for parenteral use should be rapidly available for pregnant women with a potential risk of anaphylaxis. Intramuscular adrenaline is efficient and generally well tolerated.
Adrenaline intramuscularly injected into the vastus lateralis of the quadriceps (anterolateral thigh) is the first-line treatment of anaphylaxis. Administered by intravenous route as initial treatment, adrenaline may potentially induce severe arrhythmias, even fatal as a result of bolus administration. Repeated doses of epinephrine may be needed if the symptoms are refractory to the first one.
The second-line medications in anaphylaxis may be administered only after the intramuscular injection of adrenaline. A systemic second-generation H1 antihistamine with relatively rapid onset of action, such as cetirizine, although it is not life-saving, may be used for the additional relief of pruritus and urticaria. The first-generation H1 antihistamines can cause hypotension at rapid i.v. administration and sedation which are counterproductive in anaphylaxis. A parenteral glucocorticoid, such as hydrocortisone or methylprednisolone succinate, has no immediate life-saving benefits, being administered with the intention to prevent biphasic or protracted anaphylaxis. But there is increasing evidence that systemic corticosteroids may be of no benefit in the acute management of anaphylaxis due to their slow onset of action and the potential detrimental adverse effects. Therefore, their routine use is becoming controversial(45-48).
Adrenaline studied in pregnant rhesus monkeys administered by i.v. perfusion is associated with fetal bradycardia and acidosis(49).
Even though some studies(4,21,24) have discussed the use of ephedrine as an alternative for adrenaline in pregnancy, due to its predominant beta-adrenergic effect which leads to weaker uterine vasoconstriction, adrenaline is the undisputed drug of choice, because it has a more potent effect, with a faster resolution of maternal hypotension, which ultimately leads to a better infusion of the utero-placental region(26).
When indicated, at any time, cardiopulmonary resuscitation should be performed (high-quality chest compressions may be difficult to perform in a pregnant woman) and emergency caesarean delivery should also be performed in case of anaphylaxis refractory to the medical management or for fetal distress(46).
In the context of the mentioned emergency treatment of anaphylaxis according to guidelines(6,45), it is also important to take a blood sample for the determination of serum tryptase, between 30 minutes to 4 hours after the onset of symptoms, the elevated values suggesting anaphylaxis. This is the only validated biomarker for this acute hypersensitivity reaction. Another sample should be taken at least 24 hours after the resolution of symptoms, and if serum tryptase is still significantly elevated, associated mastocytosis should be evaluated(45,46).
The quantitative measurement of total mast cell tryptase in human serum or plasma is performed by fluorescence enzyme immunoassay with capsulated cellulose polymer solid-phase. This method determines the total tryptase levels, including all forms of a-tryptase and ß-tryptase(46).
The principle of the method consists in the reaction of the tryptase in the patient sample with anti-tryptase covalently coupled to the solid phase consisting of a cellulose derivative enclosed in a capsule; ß-galactosidase-labeled antibodies against tryptase are added to form a complex incubated with a developing agent, 4-methylumbelliferyl-ß-D-galactoside, with fluorescence measurement(50,51).
There are not many case reports of anaphylaxis in pregnancy in which serum tryptase was performed. In a case of allergic anaphylaxis in late pregnancy due to amoxicillin, the plasma concentration of tryptase performed at delivery by emergency caesarean section and 120 minutes later, in both the mother and neonate, revealed normal results (serum tryptase below 11 µg/l)(52).
In a report of anaphylactic reaction due to rocuronium-sugammadex complex during elective caesarean section, the serum tryptase level was significantly elevated(53).
Serum tryptase level was also reported increased in anaphylactic shock after misoprostol in voluntary termination of pregnancy(54).
In conclusion, anaphylactic shock in pregnancy is a challenging situation for obstetrical and anesthetic team. The rapid recognition and stopping of the trigger simultaneously, with the administration of intramuscular adrenaline, may by life-saving for both mother and fetus.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
-
Hepner DL, Castells M, Mouton-Faivre C, Dewachter P. Anaphylaxis in the clinical setting of obstetric anesthesia: a literature review. Anesth Analg. 2013 Dec;117(6):1357-67. doi: 10.1213/ANE.0b013e3182a706c7.
-
Mulla ZD, Ebrahim MS, Gonzalez JL. Anaphylaxis in the obstetric patient: analysis of a statewide hospital discharge database. Ann Allergy Asthma Immunol. 2010 Jan;104(1):55-9. doi: 10.1016/j.anai.2009.11.005.
-
Jao MS, Cheng PJ, Shaw SW, Soong YK. Anaphylaxis to cefazolin during labor secondary to prophylaxis for group B Streptococcus: a case report.
-
J Reprod Med. 2006 Aug;51(8):655-658.
-
Dunn AB, Blomquist J, Khouzami V. Anaphylaxis in labor secondary to prophylaxis against group B Streptococcus. A case report. J Reprod Med. 1999 Apr;44(4):381-384.
-
McCall SJ, Bonnet MP, Äyräs O, Vandenberghe G, Gissler M, Zhang WH, Van Leeuw V, Deneux-Tharaux C, Kurinczuk JJ, Knight M; INOSS collaboration. Anaphylaxis in pregnancy: a population-based multinational European study. Anaesthesia. 2020 Nov;75(11):1469-1475. doi: 10.1111/anae.15069.
-
Simons FE, Ardusso LR, Bilò MB, El-Gamal YM, Ledford DK, Ring J, Sanchez-Borges M, Senna GE, Sheikh A, Thong BY; World Allergy Organization. World allergy organization guidelines for the assessment and management of anaphylaxis. World Allergy Organ J. 2011 Feb;4(2):13-37. doi: 10.1097/WOX.0b013e318211496c.
-
D’Astous-Gauthier K, Graham F, Paradis L, Des Roches A, Bégin P. Beta-2 Agonists May be Superior to Epinephrine to Relieve Severe Anaphylactic Uterine Contractions. J Allergy Clin Immunol Pract. 2021 Mar;9(3):1232-1241. doi: 10.1016/j.jaip.2020.10.047.
-
Berardi A, Rossi K, Cavalleri F, Simoni A, Aguzzoli L, Masellis G, Ferrari F. Maternal anaphylaxis and fetal brain damage after intrapartum chemoprophylaxis.
-
J Perinat Med. 2004;32(4):375-7. doi: 10.1515/JPM.2004.070.
-
Edmondson WC, Skilton RW. Anaphylaxis in pregnancy – the right treatment? Anaesthesia. 1994 May;49(5):454-5. doi: 10.1111/j.1365-2044.1994.tb03501.x.
-
Luciano R, Zuppa AA, Maragliano G, Gallini F, Tortorolo G. Fetal encephalopathy after maternal anaphylaxis. Case report. Biol Neonate. 1997;71(3):190-3. doi: 10.1159/000244415.
-
Cuciti C, Mayer DC, Arnette R, Spielman FJ. Anaphylactoid reaction to intravenous sodium ferric gluconate complex during pregnancy. Int J Obstet Anesth. 2005 Oct;14(4):362-4. doi: 10.1016/j.ijoa.2005.05.001.
-
Entman SS, Moise KJ. Anaphylaxis in pregnancy. South Med J. 1984 Mar;77(3):402. doi: 10.1097/00007611-198403000-00038.
-
Draisci G, Zanfini BA, Nucera E, Catarci S, Sangregorio R, Schiavino D, Mannocci A, Patriarca G. Latex sensitization: a special risk for the obstetric population? Anesthesiology. 2011 Mar;114(3):565-9. doi: 10.1097/ALN.0b013e318206ff50.
-
Deusch E, Reider N, Marth C. Anaphylactic reaction to latex during cesarean delivery. Obstet Gynecol. 1996 Oct;88(4 Pt 2):727. doi: 10.1016/0029-7844(96)00256-6.
-
Eckhout GV Jr, Ayad S. Anaphylaxis due to airborne exposure to latex in a primigravida. Anesthesiology. 2001 Oct;95(4):1034-5. doi: 10.1097/00000542-200110000-00040.
-
Draisci G, Nucera E, Pollastrini E, Forte E, Zanfini B, Pinto R, Patriarca G, Schiavino D, Pietrini D. Anaphylactic reactions during cesarean section. Int J Obstet Anesth. 2007 Jan;16(1):63-67. doi: 10.1016/j.ijoa.2006.08.006.
-
Sitarz L. Anaphylactic shock following injection of suxamethonium. Anaesth Resusc Intensive Ther. 1974 Jan-Mar;2(1):83-6.
-
Meggs WJ, Pescovitz OH, Metcalfe D, Loriaux DL, Cutler G Jr, Kaliner M. Progesterone sensitivity as a cause of recurrent anaphylaxis. N Engl J Med. 1984 Nov 8;311(19):1236-1238. doi: 10.1056/NEJM198411083111907.
-
Baraka A, Sfeir S. Anaphylactic cardiac arrest in a parturient. Response of the newborn. JAMA. 1980 May 2;243(17):1745-6.
-
Erasmus C, Blackwood W, Wilson J. Infantile multicystic encephalomalacia after maternal bee sting anaphylaxis during pregnancy. Arch Dis Child. 1982 Oct;57(10):785-787. doi: 10.1136/adc.57.10.785.
-
Gallagher JS. Anaphylaxis in pregnancy. Obstet Gynecol. 1988 Mar;71(3 Pt 2):491-493.
-
Heim K, Alge A, Marth C. Anaphylactic reaction to ampicillin and severe complication in the fetus. Lancet. 1991 Apr 6;337(8745):859-60. doi: 10.1016/0140-6736(91)92574-l.
-
Powell JA, Maycock EJ. Anaphylactoid reaction to ranitidine in an obstetric patient. Anaesth Intensive Care. 1993 Oct;21(5):702-703. doi: 10.1177/0310057X9302100539.
-
Konno R, Nagase S. Anaphylactic reaction to cefazolin in pregnancy. J Obstet Gynaecol (Tokyo 1995). 1995 Dec;21(6):577-579. doi: 10.1111/j.1447-0756.1995.tb00915.x.
-
Porter BJ, Acharya U, Ormerod AD, Herriot R. Latex/chlorhexidine-induced anaphylaxis in pregnancy. Allergy. 1998 Apr;53(4):455-457. doi: 10.1111/j.1398-9995.1998.tb03926.x.
-
Gei AF, Pacheco LD, Vanhook JW, Hankins GD. The use of a continuous infusion of epinephrine for anaphylactic shock during labor. Obstet Gynecol. 2003 Dec;102(6):1332-1335. doi: 10.1016/s0029-7844(03)00167-4. Erratum in: Obstet Gynecol. 2004 Apr;103(4):799.
-
Sheikh J. Intrapartum anaphylaxis to penicillin in a woman with rheumatoid arthritis who had no prior penicillin allergy. Ann Allergy Asthma Immunol. 2007 Sep;99(3):287-289. doi: 10.1016/S1081-1206(10)60667-9.
-
Chaudhuri K, Gonzales J, Jesurun CA, Ambat MT, Mandal-Chaudhuri S. Anaphylactic shock in pregnancy: a case study and review of the literature. Int J Obstet Anesth. 2008 Oct;17(4):350-57. doi: 10.1016/j.ijoa.2008.05.002.
-
Sleth JC, Lafforgue E, Cherici O, Nagy P. Choc anaphylactique au cours de la grossesse à terme. A propos de deux cas et revue de la littérature [Anaphylaxis in terminal pregnancy: two case studies and review of the literature]. Ann Fr Anesth Reanim. 2009 Sep;28(9):790-794. French. doi: 10.1016/j.annfar.2009.06.023.
-
Ikeda N, Oda Y, Tanaka K, Nakamura T, Asada A. [A case of anaphylactic shock induced by latex during cesarean section]. Masui. 2010 Oct;59(10):1294-1297 [Japanese].
-
Tsuzuki Y, Narita M, Nawa M, Nakagawa U, Wakai T. Management of maternal anaphylaxis in pregnancy: a case report. Acute Med Surg. 2016 Nov 10;4(2):202-204. doi: 10.1002/ams2.238.
-
Jeon HJ, Ryu A, Min J, Kim NS. Maternal anaphylactic shock in pregnancy: A case report. Medicine (Baltimore). 2018 Sep;97(37):e12351. doi: 10.1097/MD.0000000000012351.
-
Takahashi M, Hotta K, Inoue S, Takazawa T, Horiuchi T, Igarashi T, Takeuchi M. Mepivacaine-induced anaphylactic shock in a pregnant woman undergoing combined spinal and epidural anesthesia for cesarean delivery: a case report. JA Clin Rep. 2019 Dec 19;5(1):84. doi: 10.1186/s40981-019-0302-6.
-
Cousins L. Fetal oxygenation, assessment of fetal well-being, and obstetric management of the pregnant patient with asthma. J Allergy Clin Immunol. 1999 Feb;103(2 Pt 2):S343-349. doi: 10.1016/s0091-6749(99)70260-5.
-
Lombaard H, Soma-Pillay P, Farrell el-M. Managing acute collapse in pregnant women. Best Pract Res Clin Obstet Gynaecol. 2009 Jun;23(3):339-355. doi: 10.1016/j.bpobgyn.2009.01.005.
-
Suresh MS, LaToya Mason C, Munnur U. Cardiopulmonary resuscitation and the parturient. Best Pract Res Clin Obstet Gynaecol. 2010 Jun;24(3):383-400. doi: 10.1016/j.bpobgyn.2010.01.002.
-
Adriaensens I, Vercauteren M, Soetens F, Janssen L, Leysen J, Ebo D. Allergic reactions during labour analgesia and caesarean section anaesthesia. Int J Obstet Anesth. 2013 Jul;22(3):231-242. doi: 10.1016/j.ijoa.2013.04.010.
-
Piccinni MP. T-cell cytokines in pregnancy. Am J Reprod Immunol. 2002 May;47(5):289-94. doi: 10.1034/j.1600-0897.2002.01104.x.
-
Krishnan L, Guilbert LJ, Russell AS, Wegmann TG, Mosmann TR, Belosevic M. Pregnancy impairs resistance of C57BL/6 mice to Leishmania major infection and causes decreased antigen-specific IFN-gamma response and increased production of T helper 2 cytokines. J Immunol. 1996 Jan 15;156(2):644-652.
-
Chaouat G, Assal Meliani A, Martal J, Raghupathy R, Elliott JF, Mosmann T, Wegmann TG. IL-10 prevents naturally occurring fetal loss in the CBA x DBA/2 mating combination, and local defect in IL-10 production in this abortion-prone combination is corrected by in vivo injection of IFN-tau. J Immunol. 1995 May 1;154(9):4261-4268. Erratum in: J Immunol. 2005 Sep 1;175(5):3447. Elliot, J [corrected to Elliott, JF].
-
Markert UR, Arck PC, Peiker G, Mock BA. Might wasp venom desensitization induced Th2 to Th1 shift cause pregnancy failure? Am J Reprod Immunol. 2002 Apr;47(4):193-195. doi: 10.1034/j.1600-0897.2002.01063.x.
-
Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012 May 4;18(5):693-704. doi: 10.1038/nm.2755.
-
Platts-Mills TAE, Schuyler AJ, Erwin EA, Commins SP, Woodfolk JA. IgE in the diagnosis and treatment of allergic disease. J Allergy Clin Immunol. 2016 Jun;137(6):1662-1670. doi: 10.1016/j.jaci.2016.04.010.
-
Kraft S, Kinet JP. New developments in FcepsilonRI regulation, function and inhibition. Nat Rev Immunol. 2007 May;7(5):365-378. doi: 10.1038/nri2072.
-
Cardona V, Ansotegui IJ, Ebisawa M, El-Gamal Y, Fernandez Rivas M, Fineman S, Geller M, Gonzalez-Estrada A, Greenberger PA, Sanchez Borges M, Senna G, Sheikh A, Tanno LK, Thong BY, Turner PJ, Worm M. World allergy organization anaphylaxis guidance 2020. World Allergy Organ J. 2020 Oct 30;13(10):100472.
-
Simionescu AA, Stănescu AMA, Popescu FD. State-of-the-Art on Biomarkers for Anaphylaxis in Obstetrics. Life (Basel). 2021 Aug 24;11(9):870. doi: 10.3390/life11090870.
-
Anchor J, Settipane RA. Appropriate use of epinephrine in anaphylaxis. Am J Emerg Med. 2004 Oct;22(6):488-90. doi: 10.1016/j.ajem.2004.07.016.
-
Campbell DE. Anaphylaxis Management: Time to Re-Evaluate the Role of Corticosteroids. J Allergy Clin Immunol Pract. 2019 Sep-Oct;7(7):2239-2240.
-
Adamsons K, Mueller-Heubach E, Myers RE. Production of fetal asphyxia in the rhesus monkey by administration of catecholamines to the mother. Am J Obstet Gynecol. 1971 Jan 15;109(2):248-62. doi: 10.1016/0002-9378(71)90873-8.
-
Sheldon J, Philips B. Laboratory investigation of anaphylaxis: not as easy as it seems. Anaesthesia. 2015 Jan;70(1):1-5. DOI: 10.1111/anae.12926.
-
Popescu FD, Vieru M. Precision medicine allergy immunoassay methods for assessing immunoglobulin E sensitization to aeroallergen molecules. World J Methodol. 2018 Nov 29;8(3):17-36.
-
Sleth JC. Anaphylaxis in late pregnancy: plasma concentrations of histamine, tryptase and IgE in the neonate. Int J Obstet Anesth. 2018 Nov;36:138-139. doi: 10.1016/j.ijoa.2018.02.007.
-
Yamaoka M, Deguchi M, Ninomiya K, Kurasako T, Matsumoto M. A suspected case of rocuronium-sugammadex complex-induced anaphylactic shock after cesarean section. J Anesth. 2017 Feb;31(1):148-151.
-
Béné J, Alarcon P, Faucon M, Auffret M, Delfosse F, Becker T, De Zorzi S, Gautier S. Anaphylactic shock after misoprostol in voluntary termination of pregnancy – a case report. Eur J Obstet Gynecol Reprod Biol. 2014 Nov;182:260-261.
Articole din ediţiile anterioare
Rolul 25 hidroxivitaminei D (25(OH)D) în travaliul prematur
Travaliul prematur este cea mai importantă cauză de deces în cazul copiilor sub 5 ani, la nivel mondial. Etiologia travaliului prematur este adesea...
Creşterea progesteronului ca declanşator al ovulaţiei
Deşi există numeroase studii care au dezbătut fiziologia procesului de ovulaţie, implicând anumiţi hormoni cu roluri bine determinate, studiile rec...
COVID-19 în sarcină – dificultăţi şi progrese
The newly emerging coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is spreading gl...
Condyloma acuminata în timpul sarcinii
As counted by the Centers for Disease Control and Prevention (CDC), anogenital human papillomavirus (HPV) is the most common and widespread sexuall...