We aim to present an adult female confirmed with severe hypothyroidism due to Hashimoto’s autoimmune thyroiditis that was associated with hyperprolactinemia which, combined, led to secondary amenorrhea. Additionally, hypercholesterolemia, anemia and vitamin D deficiency were confirmed, and the patient’s electrocardiogram showed flattened T waves. The value of TSH (thyroid stimulating hormone) was unexpectedly high, approximately 100 times upper the normal limit. The patient experienced a progression of clinical manifestations within one year, while she did not seek for medical care. This unexpected severity of thyroid hormones deficiency is rarely found nowadays due to active screening protocols and easy access to thyroid assessment; however, in young individuals, the clinical tolerance to myxedema-associated issues is higher than seen in seniors, thus a delay of diagnosis might be found. Whether this impacts the overall reproductive potential in young females mostly depends on the compliance to lifelong hormonal replacement and surveillance of adequate serum hormonal levels, including during pregnancy.
Severe primary hypothyroidism-related hyperprolactinemia and secondary amenorrhea
Hipotiroidism primar sever asociat cu hiperprolactinemie şi amenoree secundară
First published: 22 decembrie 2023
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/ObsGin.71.4.2023.9133
Abstract
Rezumat
Prezentăm cazul unei tinere paciente confirmate cu hipotiroidism sever legat de tiroidita autoimună Hashimoto, asociat cu hiperprolactinemie, care, toate combinate, au condus la amenoree secundară. Adiţional, au fost detectate hipercolesterolemie, anemie şi hipovitaminoză D, iar electrocardiograma a arătat unde T aplatizate. Valoarea TSH-ului (hormonul de stimulare tiroidiană) a fost neaştepat de mare, de aproximativ 100 de ori peste limita superioară a normalului. Pacienta a prezentat progresia manifestărilor clinice în decursul unui an, timp în care nu a fost evaluată medical. Acest nivel sever al deficitului hormonilor tiroidieni este rar identificat în prezent, în contextul protocoalelor de screening active şi al accesului facil la bilanţul tiroidian. Totuşi, la persoanele tinere, toleranţa clinică a mixedemului este mai mare decât la seniori, de aceea poate avea loc o întârziere a diagnosticului. Dacă acesta afectează potenţialul reproductiv la femeile tinere depinde cel mai mult de complianţa la substituţia hormonală pe parcursul vieţii şi de menţinerea unui nivel adecvat al hormonilor, inclusiv pe perioada unei sarcini.
1. Introduction
The most frequent cause of hypothyroidism in iodine-sufficient regions is chronic autoimmune thyroiditis, commonly referred to as Hashimoto’s disease, which causes inadequate synthesis and release of thyroid hormones(1). The disease is more common in women, and the prevalence of Hashimoto’s disease varies by area and socioeconomic status; in women, it ranges from 4.8% to 25.8%, and in men, from 0.9% to 7.9%(2). Autoimmune thyroiditis is characterized by a particular lymphocytic infiltration of the parenchyma belonging to the thyroid gland and the presence of particular antibodies, mainly antithyroglobulin (ATG) antibodies and antithyroperoxidase (ATPO) antibodies(3).
Globally, hypothyroidism has a wide spectrum of clinical manifestations and frequently appears as menses anomalies and reproductive potential impairment in addition to a wide area of clinical elements such as tiredness (81%), dry skin (63%), puffy eyes, depressive symptoms, reduced quality of life, altered cognitive function and memory, weight gain, constipation, increased sensitivity to cold (cold intolerance), reduced hearing, and even voice changes(4-6).
These clinical features are tidily related to the thyroid hormones-associated roles, since they are important regulators of the metabolic, vascular and cardiac physiology. Therefore, deficiency of thyroid hormones affects the body in many ways, particularly in the cardiovascular system, where they can have a major impact on heart function, with an increased risk for atherosclerosis(7). Hence, hypothyroidism is associated with different abnormalities in electrocardiography such as delayed intraventricular conduction, flattened P waves, flat or inverted T waves and, traditionally, sinus bradycardia(8). Also, hypothyroidism is characterized by a decrease in the expression of liver LDL (low-density lipoproteins) receptors and a reduction in the activity of cholesterol-a-monooxygenase, which breaks down cholesterol, resulting in an overall decreased LDL clearance(9). Therefore, dyslipidemia, particularly hypercholesterolemia, is frequently associated with hypothyroidism and with potential negative effects at the cardiovascular and metabolic level(10). Anemia can be associated with thyroid dysfunction, particularly hypothyroidism, and it impairs the pregnancy outcome in selected (severe) female cases(11). Many studies found a correlation between hypothyroidism and vitamin D levels, but, overall, females with vitamin D deficiency are quite frequently found among the population confirmed with low levels of thyroid hormones, particularly primary type of hypothyroidism/myxedema(12).
The diagnosis of hypothyroidism requires levothyroxine replacement therapy as the standard care; additionally, the decision of lipid lowering drugs for hypercholesterolemia or estroprogestive in cases with myxedema-related amenorrhea is based on individual management, since restoring normal thyroid hormones levels is expected to improve many related aspects(13,14).
We aim to present an adult female confirmed with severe hypothyroidism due to Hashimoto’s thyroiditis that was associated with hyperprolactinemia which, combined, led to secondary amenorrhea. Additionally, hypercholesterolemia, anemia and vitamin D deficiency were confirmed. The patient’s electrocardiogram showed flattened T waves which were very suggestive of myxedema. This unexpected severity of thyroid hormones deficiency is rarely found nowadays due to active screening protocols and easy access to thyroid assessment; however, in young individuals, the clinical tolerance to associated issues is higher than seen in seniors, thus a delay of diagnosis might be found. Whether this impacts the overall reproductive potential in young females mostly depends on the compliance to lifelong hormonal replacement and surveillance of adequate serum hormonal levels, including during pregnancy.
2. Case presentation
A 26-year-old lady, coming from an endemic area, with no family history of any endocrine pathology, was admitted for a secondary checkup following the detection of an abnormally high TSH (thyroid stimulating hormone) level (above 500 µUI/mL, meaning approximately 100 times increase upper the normal limit), a value that was found during a routine testing.
During examination, the patient revealed typical symptoms and indicators of hypothyroidism. The patient complained of generalized weakness, constipation, hair loss, depression, excessive sleepiness, anorexia, reduced hearing and secondary amenorrhea (she did not take any progestative, neither estroprogestative; she had a negative pregnancy test on current admission). She reported that the symptoms had progressed over the course of a year, and no specific medical therapy was initiated. Medical family history revealed type 1 diabetes mellitus in her mother. The clinical examination of the patient revealed normal vital signs, blood pressure of 113/82 mmHg, and pulse of 60-65 per minute (regular). Her weight was 67 kg and her height was 162 cm, with a Body Mass Index of 25.53 kg/m2 and clinical signs of myxedema: minimal periorbital edema, coarse hair, dry skin, and yellow palmar discoloration consistent with hypercarotenemia (Figure 1).
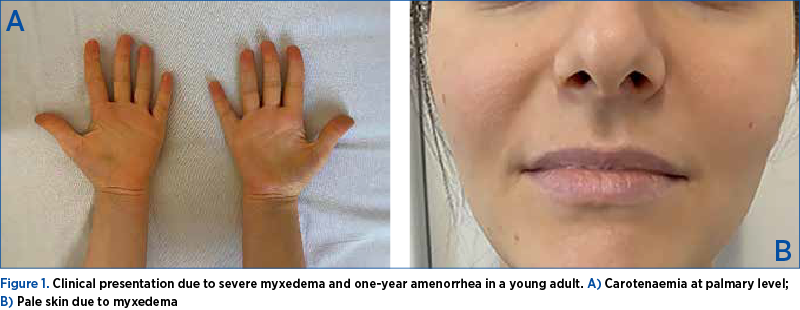
The thyroid gland was not palpable, and no surficial cervicolateral lymphadenopathy was detected. She had a menarche at 12 years old, and normal menstrual cycle until one year prior (nulipara).
The laboratory findings started with the first endocrine assessments, showing a TSH value above 500 µUI/mL (normal range between 0.27 and 4.2 µUI/mL) and fT4 (free levothyroxine) below 0.5 pmol/L (normal range between 12 and 22 pmol/L) in association with increased hepatic enzymes in terms of AST (aminotransferase) of 41 UI/L (normal range: below 35 UI/L). The current laboratory evaluation also revealed hypercholesterolemia: total cholesterol of 558 mg/dL (normal range between 0 and 200 mg/dL), LDL-cholesterol of 475 mg/dL (normal range: 60-160 mg/dL); normochromic macrocytic anemia in terms of hemoglobin of 11.1 g/dL (normal range: 12-15.5 g/dL) while being in secondary amenorrhea, as already mentioned (Table 1).
Thyroid evaluation showed (once again) severe hypothyroidism, with a low FT4 level of 5.8 pmol/L (normal range: 9-19 pmol/L) with extremely high TSH, above 100 µUI/mL (normal range: 0.35-4.94 µUI/mL; of note, this was a different lab to her prior assays). A subsequent ATPO testing showed high levels of 126.6 UI/mL (normal range: 0-5.61 UI/mL) with normal ATG. A mild hyperprolactinemia was detected, namely a serum prolactin of 40.79 ng/dL (normal range: 3.34-26.72 ng/mL). Plasma morning ACTH (adrenocorticotropic hormone) and cortisol levels were normal, and no further dynamic test was necessary to confirm the normal adrenal function. Hypovitaminosis D was also detected in terms of 25-hydroxyvitamin D of 19.8 ng/mL (normal range: above 30 ng/mL) without secondary hyperparathyroidism (Table 2).
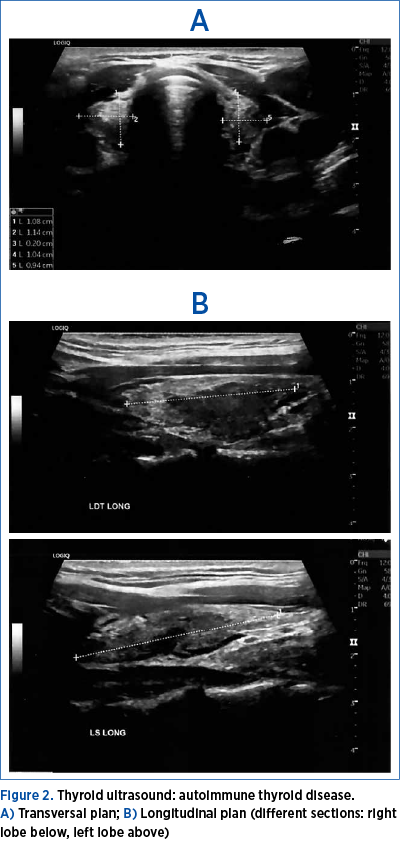
Thyroid ultrasound showed a hypoechoic pattern suggestive of a thyroid autoimmune disease (Figure 2).
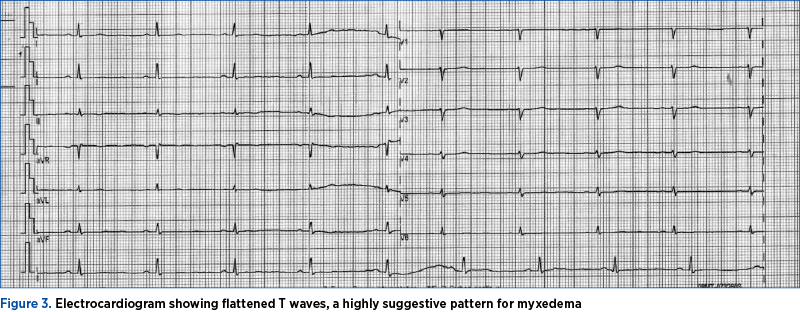
We also performed a cardiologic evaluation, the electrocardiogram revealing flattened T waves, suggestive of myxedema, while the cardiac ultrasound showed no pericardial liquid (Figure 3).
Oral administration of daily levothyroxine was offered to the young lady, 50 µg/day for the first week, followed by a gradual increase to 100 µg/day; the first checkup of TSH and FT4 was after 6-8 weeks. Soon after the initiation of the thyroid hormone replacement, the menses spontaneously restarted. Further hematologic assessments revealed no particular cause of anemia, thus the reduced general metabolic rate amidst myxedema was considered as the single cause. Life-long surveillance of levothyroxine substitution is required with periodic assays. In case of pregnancy, an increase dose is mandatory. A higher risk than in the general population in order to associate other endocrine autoimmune conditions such as primary adrenal failure or premature ovarian insufficiency is estimated, thus awareness is necessary. Moreover, 2000 UI of daily cholecalciferol was initiated for three months, followed by another three months of 1000 UI/day.
3. Discussion
This case highlights several aspects that are worth being mentioned.
Firstly, primary hypothyroidism leads to increased thyrotropin-releasing hormone (TRH) release from the hypothalamus, which has a trophic effect on thyrotrophs and lactotrophs cells, resulting in increased TSH and prolactin levels. This may potentially lead to pituitary gland enlargement, too. In most severe cases, primary hypothyroidism/myxedema has mild hyperprolactinemia(15).
Hyperprolactinemia is commonly caused by primary hypothyroidism; however, not all patients experience this condition. Of those with overt primary hypothyroidism, up to 40% of patients have been reported to have hyperprolactinemia(16,17). Long-term hyperprolactinemia can cause hypogonadism and related irregular menstruation, secondary amenorrhea, sexual dysfunction, and even low bone mineral density, osteoporosis and fragility fractures in the long term(18-20).
Another aspect relates to the importance of autoimmune background in a female of reproductive age due to a higher risk (of four to ten times) of developing another endocrine autoimmune condition, especially Addison’s disease or premature ovarian failure but, also, non-endocrine pathologies such as vitiligo or lupus etc.(21,22)
Anemia in autoimmune hypothyroidism might be caused by the low level of thyroid hormones as a direct consequence or associated autoimmune anomalies at gastric or intestinal level, also causing B12 vitamin deficiency; alternatively, an incidental cause such as anorexia or minor beta-thalassemia should be taken into consideration(23,24).
Another important aspect is the admission of a patient with a large area of myxoedema-associated complications that developed them within one year via a progressive onset. Of course, we may conclude that delayed presentation might be related to the medical and social issue that came with a late COVID-19 pandemic reality, as seen in other medical areas, including the thyroid field(25,26). However, the prognosis was good due to prior irrelevant comorbidities and compliance to the recommendations once the diagnosis was established.
Further on, the young lady was seeking for fertility, and the long-term outcome seems good if hormonal replacement therapy is continued and adjusted over time. The therapeutic use of levothyroxine in pregnancy and newborn is well recognized, with good maternal-fetal outcomes. Controversies around the target TSH amidst gestation are still present. The best surveillance hormone is FT4, not TSH, due to pregnancy-related effects of the maternal estrogens in binding proteins and thyroid hormones-TSH feedback(27,28). Another aspect to be followed is the correction of hypovitaminosis D to improve the general health, but also to ensure an optimal evolution of the mineral metabolism, including over the pregnancy(29,30).
4. Conclusions
The late presentation for myxedema complicated with hyperprolactinemia in a young female, associating a large panel of clinical elements, including secondary amenorrhea that did not require a specific intervention, apart from levothyroxine substitution. A particular contribution to fertility profile and future pregnancy also involves the diagnosis of vitamin D deficiency.
Abbreviations: ACTH = adrenocorticotropic hormone; ALT = aminotransferase; AST = alanine aminotransferase; ATG = antithyroglobulin antibodies; ATPO = antithyroid peroxidase; FT4 = free levothyroxine; TSH = thyroid stimulating hormone; 25-OHD = 25-hydroxyvitamin D
Acknowledgement. We thank the patient for her written consent to present her medical records and above displayed captures.
Corresponding author: Mara Carsote, e-mail: carsote_m@hotmail.com
Conflict of interest: none declared
Financial support: none declared
This work is permanently accessible online free of charge and published under the CC-BY.

Bibliografie
-
Chaker L, Bianco AC, Jonklaas J, Peeters RP. Hypothyroidism. Lancet. 2017;390(10101):1550-1562.
-
Hu X, Chen Y, Shen Y, Tian R, Sheng Y, Que H. Global prevalence and epidemiological trends of Hashimoto’s thyroiditis in adults: A systematic review and meta-analysis. Front Public Health. 2022;10:1020709.
-
Zamwar UM, Muneshwar KN. Epidemiology, Types, Causes, Clinical Presentation, Diagnosis, and Treatment of Hypothyroidism. Cureus. 2023;15(9):e46241.
-
Jansen HI, Boelen A, Heijboer AC, Bruinstroop E, Fliers E. Hypothyroidism: The difficulty in attributing symptoms to their underlying cause. Front Endocrinol (Lausanne). 2023;14:1130661.
-
Chaker L, Razvi S, Bensenor IM, Azizi F, Pearce EN, Peeters RP. Hypothyroidism. Nat Rev Dis Primers. 2022;8(1):30.
-
Larsen CB, Winther KH, Cramon PK, Rasmussen ÅK, Feldt-Rasmussen U, Groenvold M, Bjorner JB, Hegedüs L, Watt T, Bonnema SJ. Severity of hypothyroidism is inversely associated with impaired quality of life in patients referred to an endocrine clinic. Thyroid Res. 2023;16(1):37.
-
Stamatouli A, Bedoya P, Yavuz S. Hypothyroidism: Cardiovascular Endpoints of Thyroid Hormone Replacement. Front Endocrinol (Lausanne). 2020;10:888.
-
Tayal B, Graff C, Selmer C, Kragholm KH, Kihlstrom M, Nielsen JB, Olsen AS, Pietersen AH, Holst AG, Søgaard P, Christiansen CB, Faber J, Gislason GH, Torp-Pedersen C, Hansen SM. Thyroid dysfunction and electrocardiographic changes in subjects without arrhythmias: a cross-sectional study of primary healthcare subjects from Copenhagen. BMJ Open. 2019;9(6):e023854.
-
Jabbar A, Pingitore A, Pearce SH, Zaman A, Iervasi G, Razvi S. Thyroid hormones and cardiovascular disease. Nat Rev Cardiol. 2017;14(1):39-55.
-
Mavromati M, Jornayvaz FR. Hypothyroidism-Associated Dyslipidemia: Potential Molecular Mechanisms Leading to NAFLD. Int J Mol Sci. 2021;22(23):12797.
-
Soliman AT, De Sanctis V, Yassin M, Wagdy M, Soliman N. Chronic anemia and thyroid function. Acta Biomed. 2017;88(1):119-127.
-
Czarnywojtek A, Florek E, Pietrończyk K, Sawicka-Gutaj N, Ruchała M, Ronen O, Nixon IJ, Shaha AR, Rodrigo JP, Tufano RP, Zafereo M, Randolph GW, Ferlito A. The Role of Vitamin D in Autoimmune Thyroid Diseases: A Narrative Review. J Clin Med. 2023;12(4):1452.
-
McDermott MT. Hypothyroidism. Ann Intern Med. 2020;173(1):ITC1-ITC16.
-
Jonklaas J. Update on the treatment of hypothyroidism. Curr Opin Oncol. 2016;28(1):18-25.
-
Sheikhi V, Heidari Z. Increase in Thyrotropin Is Associated with an Increase in Serum Prolactin in Euthyroid Subjects and Patients with Subclinical Hypothyroidism. Med J Islam Repub Iran. 2021;35:167.
-
Sharma LK, Sharma N, Gadpayle AK, Dutta D. Prevalence and predictors of hyperprolactinemia in subclinical hypothyroidism. Eur J Intern Med. 2016;35:106-110.
-
Vilar L, Vilar CF, Lyra R, Freitas MDC. Pitfalls in the Diagnostic Evaluation of Hyperprolactinemia. Neuroendocrinology. 2019;109(1):7-19.
-
Fukuhara N, Nishiyama M, Iwasaki Y. Update in Pathogenesis, Diagnosis, and Therapy of Prolactinoma. Cancers (Basel). 2022;14(15):3604.
-
Koyyada A, Orsu P. Role of hypothyroidism and associated pathways in pregnancy and infertility: Clinical insights. Tzu Chi Med J. 2020;32(4):312-317.
-
Nappi RE, Di Ciaccio S, Genazzani AD. Prolactin as a neuroendocrine clue in sexual function of women across the reproductive life cycle: an expert point of view. Gynecol Endocrinol. 2021;37(6):490-496.
-
Şandru F, Cârşote M, Albu SE, Dumitraşcu MC, Valea A. Vitiligo and chronic autoimmune thyroiditis. J Med Life. 2021;14(2):1-4.
-
Şandru F, Petca A, Dumitraşcu MC, Petca RC, Cârşote M. Peutz-Jeghers syndrome: skin manifestations and endocrine anomalies (Review). Exp Ther Med. 2021:22(6):1387.
-
Safavi Ardabili N, Rahimi F, Ranjbar A, Montazeri F, Darsareh F. Maternal and Neonatal Outcomes of Sub-clinical Hypothyroidism Treated with Levothyroxine in Pregnancy: A Retrospective Cohort Study. Cureus. 2023;15(9):e45352.
-
Dumitraşcu MC, Şandru F, Cârşote M, Petca RC, Gheorghisan-Gălăţeanu AA, Petca A, Valea A. Anorexia nervosa: COVID-19 pandemic period (Review). Exp Ther Med. 2021;22(2):804.
-
Popescu M, Ghemigian A, Vasile CM, Costache A, Cârşote M, Ghenea AE. The new entity of subacute thyroiditis amid the COVID-19 pandemic: from infection to vaccine. Diagnostics (Basel). 2022:12(4):960.
-
Şandru F, Cârşote M, Petca RC, Gheorghisan-Gălăţeanu AA, Petca A, Valea A, Dumitraşcu MC. COVID-19-related thyroid conditions (Review). Exp Ther Med. 2021;22(1):276.
-
Urgatz B, Poppe KG. Update on therapeutic use of levothyroxine for the management of hypothyroidism during pregnancy. Endocr Connect. 2024:EC-23-0420.
-
Feldt-Rasmussen U, Effraimidis G, Bliddal S, Klose M. Risks of suboptimal and excessive thyroid hormone replacement across ages. J Endocrinol Invest. Published online November 28, 2023.
-
Paschou SA, Bletsa E, Papazisi M, Mili N, Kanouta F, Kassi GN, Psaltopoulou T, Goulis DG, Lambrinoudaki I. Screening and management of major endocrinopathies during pregnancy: an update. Endocrine. 2023;80(1):10-19.
-
Cyprian F, Lefkou E, Varoudi K, Girardi G. Immunomodulatory Effects of Vitamin D in Pregnancy and Beyond. Front Immunol. 2019;10:2739.
Articole din ediţiile anterioare
Pituitary neuroendocrine tumors (PitNET)-related menses peculiarities
Prolactinoamele sunt tumori neuroendocrine pituitare (PitNET) interesante. Dintre toate aceste tumori, prolactinoamele reprezintă 30-50%, de obicei...