Recurrent implantation failures have multiple causes. Microorganisms frequently invade the uterine cavity, and consecutive endometrial infection or inflammation can play a role in implantation failure during assisted human reproduction techniques. Pathological microorganisms and inflammatory mediators in the host can cause a cascade of events, leading to the implantation failure or expulsion of the embryo. This review addresses an important factor related to implantation failure, dysbiosis and chronic endometritis. Prolonged menstrual bleeding, previous abortions, uterine curettage, tubal pathology or a history of vaginal bacterial infections are common risk factors for endometritis. Chronic endometritis is often asymptomatic, but it plays an important role in implantation failures. The embryo implantation rates were significantly higher in patients receiving combined antibiotic therapy for chronic endometritis. It is therefore important that, in the case of infertile couples, this potential cause be sought and treated, because, if left untreated, it has the potential to determine recurrent implant failure. Oral antibiotic therapy, associated with oral and vaginal probiotics, plays an important role in regulating endometrial microbiome.
The importance of chronic endometritis and dysbiosis in implantation failure in IVF cycles
Importanţa endometritei cronice şi a disbiozei în eşecul de implantare în ciclurile de FIV
First published: 30 iunie 2023
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/ObsGin.71.2.2023.8877
Abstract
Rezumat
Eşecurile recurente de implantare au cauze multiple. Cavitatea uterină este frecvent invadată de microorganisme, iar infecţia sau inflamaţia endometrială pot juca un rol în eşecul de implantare în cadrul tehnicilor de reproducere umană asistată. Microorganismele patologice şi mediatorii inflamatori ai gazdei pot provoca o cascadă de evenimente care duc la eşecul de implantare sau la expulzarea embrionului. Acest review al noutăţilor din literatură abordează un factor important legat de eşecul de implantare, disbioză şi endometrita cronică. Sângerarea menstruală prelungită, avorturile anterioare, chiuretajul uterin, patologia tubară şi istoricul infecţiilor bacteriene vaginale sunt factori de risc obişnuiţi pentru endometrită. Endometrita cronică este adesea asimptomatică, dar joacă un rol important în eşecurile de implantare. Ratele de implantare a embrionilor au fost semnificativ mai mari la pacientele care au primit terapie cu antibiotice combinate pentru endometrita cronică. Prin urmare, este important ca, în cazul cuplurilor infertile, această cauză potenţială să fie investigată şi tratată, deoarece, dacă este lăsată netratată, are potenţialul de a determina eşecul recurent al implantării. Terapia cu antibiotice orale, asociată cu probiotice orale şi vaginale, joacă un rol important în reglarea microbiomului endometrial.
Introduction
We often face in vitro fertilization cycles in which embryo implantation does not occur despite the apparent exclusion of other maternal unfavorable conditions. Then, we start to investigate why the implantation did not occur. There are many causes for the implantation failure of an embryo, and these are related to either the embryo, or the maternal organism. If we consider the quality of embryos, we do not expect an embryo with a higher fragmentation and a lower quality to evolve. Still, an embryo of the best quality has a much higher chance of implantation than an embryo with accentuated fragmentation and poor quality. There are situations when we have the best-quality embryos, but the implantation does not occur in the end. In these cases, the main factor is “the host”, meaning the maternal uterus, where several causes “contribute” to the implant failure. This review of current literature addresses chronic endometritis and dysbiosis of the uterine cavity.
Chronic endometritis. The pathogenesis of chronic endometritis is related to an impairment of the endometrial microbiome and abnormal proliferation of various types of microorganisms, mainly Gram-negative bacteria (Enterococcus faecalis, Mycoplasma, Ureaplasma, Chlamydia, Escherichia coli and Streptococcus spp.)(1,2,3,4,5). This pathology changes the quality of the endometrium. It forms an inflammatory infiltrate that, if left untreated, can lead to implant failure(6,7). Romero et al. reported in one study that 15% of infertile women who resorted to an in vitro fertilization (IVF) procedure had chronic endometritis, and in 42% of women with implant failure, chronic endometritis was diagnosed after hysteroscopic biopsy(8). Another recent study published by Zolghadri et al. reports the incidence of chronic endometritis in women with three or more recurrent pregnancy losses in 57% of cases(9).
A recently published retrospective study, performed on 446 patients diagnosed with infertility, in whom hysteroscopy was performed with endometrial biopsies, showed that approximately 43% of these were diagnosed with asymptomatic chronic endometritis(10).
Dysbiosis represents the alteration of the endometrial microbiota. There should be a specific microbiota in the uterine cavity, as in any body cavity. In the uterine cavity, lactobacilli must predominate, which should occupy over 90% of the present microorganisms(11). Endometrial quality is affected if lactobacilli are in small amounts, and implantation failure may occur(12). A recent meta-analysis concluded that women who perform IVF procedures and have an altered endometrial microbiome are 1.4 times less likely to have a successful embryo nidation than patients with normal endometrial microbiome(13). To determine the endometrial microbiome, a biopsy is performed, using a Pipelle cannula in the endometrial cavity, in the luteal phase P+5.
Presentation of a revelatory case
In the following, we present a clinical case of recurrent implantation failures following fifth-day embryo transfers.
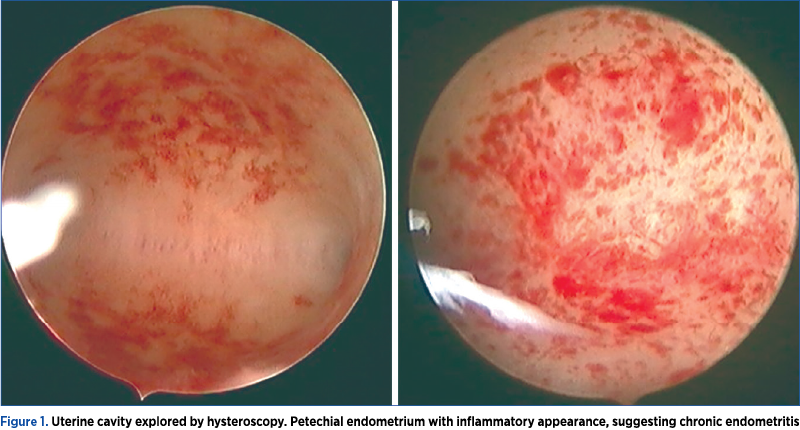
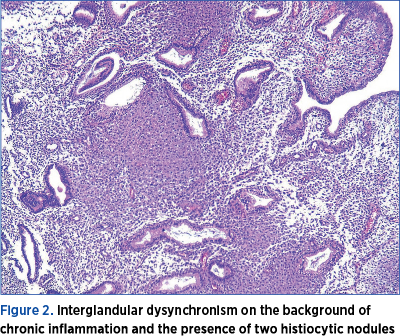
A 28-year-old woman, diagnosed with tubal infertility, performed an IVF procedure in which five fifth-day embryos were obtained. The woman performed five embryo transfers with no positive beta-HCG. After the first failed embryo transfer, hysteroscopy was performed, where the uterine cavity was found with inflammatory-looking petechial areas (Figure 1), from which fragments were taken and sent to anatomic pathology for histopathological examination. The histopathological results revealed chronic lymphocytic inflammatory infiltrates (Figure 2). The patient received oral antibiotic treatment, doxycycline 100 mg, every 12 hours, for seven days. Another four unsuccessful embryo transfers were performed.
The patient was addressed to our center, where we started a new stimulation procedure only after we performed endometrium genetic testing of the endometrial microbiome, genetic testing for its immunology, and genetic testing for endometrial receptivity. A biopsy was performed according to the laboratory indications in the luteal phase (P+5) and revealed a low percentage of lactobacilli (57%) in the endometrial microbiome. No bacterial species that can cause chronic endometritis were identified. Other microorganisms were highlighted: Micrococcaceae in the proportion of 15%, Burkholderiales – 16%, and others – 11%. Following this result, oral and vaginal antibiotic and probiotic treatment was recommended. According to the literature, we treated dysbiosis with oral antibiotics and oral and vaginal probiotics(14). We recommended to the patient an antibiotic treatment with quinolones and metronidazole (ofloxacin and metronidazole) for 10 days, associated with oral and vaginal probiotics (Lactobacillus crispatus, rhamnosus, gasseri, jensenii, acidophilus, plantarum).
Genetic testing of endometrial immunology revealed a decrease in natural killer (NK) cells associated with a significant increase in Th1 lymphocytes. There are recommendations for the imbalance between NK cells and T lymphocytes. Accordingly, in the controlled cycle, we administered three recombinant HCG vials on days P+4, P+6 and P+8(15,16,17,18).
Genetic testing for endometrial receptivity revealed normal endometrial receptivity of the endometrium.
Following the treatments presented before, the fertilization procedure was performed. The first transfer was made with a frozen embryo on day P+5, and the result was the birth of a male sexual fetus, weighing 2780 g, with an Apgar score of 8. The birth occurred at 36 weeks due to premature rupture of the membranes.
Discussion
The proper diagnosis of endometrial cavity conditions in infertile patients and their hysteroscopic treatment can resolve the cause of infertility(5). For example, hysteroscopic polypectomy provided better results for couples who performed an IVF procedure(6,19). Similarly, treating adenomyosis, malformations, hydrosalpinx, intracavitary or submucous leiomyoma may help(20,21,22). Endometrial receptivity testing did not show a significant utility over time, because it has not demonstrated an increase in the rate of live births in patients tested for the implant window(6).
Endometritis is defined as the inflammation of the endometrium, which can be caused by many factors. Prolonged menstrual bleeding, previous abortions, uterine curettage, tubal pathology or a history of vaginal bacterial infections are common risk factors for endometritis(23,24).
Chronic endometriosis can manifest in different clinical forms, depending on the causal factor or the symptoms presented by the patient(25). This is a condition difficult to diagnose. Vaginal ultrasound examinations or clinical examinations cannot detect it. Chronic endometritis can only be suspected in rare cases where complications occur: synechia, hydrometra or pyometra(26). Although chronic endometritis is often asymptomatic(5,6), it plays an important role in implantation failures. In Romero’s study, it was shown that 15% of infertile women who resorted to an IVF procedure had chronic endometritis, and in 42% of women with implant failure, chronic endometritis was diagnosed following the hysteroscopic biopsy(8). It is therefore important that, in the case of infertile couples, this potential cause be sought and treated, because, if left untreated, it may lead to recurrent implant failure(1,15).
Chronic endometritis has not been studied in fertile patients. Rather, it was investigated following a diagnosis of infertility. In the most recently published study of 446 patients diagnosed with infertility of various causes, an endometrial biopsy was performed during the hysteroscopy before the IVF procedure, and 192 patients (43%) were diagnosed with chronic endometriosis following hysteroscopic appearance and histopathological examination(10).
In the same study, the implantation rates in patients receiving combined antibiotic therapy were significantly higher (43.2%) than in the group where antibiotherapy had not been administered (27.3%).
The group of Cicinelli et al. studied the effect of antibiotic monotherapy on patients diagnosed with chronic endometritis and the implantation rate after antibiotic treatment.
If chronic endometritis and Gram-negative bacteria had been identified, ciprofloxacin 500 mg was administered at 12 hours for 10 days; if Gram-positive bacteria had been identified, they administered amoxicillin and clavulanic acid 1 g, at 12 hours, for eight days. Following the treatment regimen, the authors verified the persistence of endometritis by hysteroscopy. The rate of live births in patients who responded to antibiotic treatment was 61% compared to 13% in women who did not respond to antibiotic treatment(27).
Antibiotic treatment with doxycycline 100 mg, two tablets per day, combined with metronidazole 500 mg, two tablets per day, for 14 days, resulted in the treatment of chronic endometritis at a rate of 75% in the case of a study published by McQueen in 2014. Also, in this study, the rate of live births was significantly higher in the group treated with combined antibiotic therapy (56%), compared to 7% in the group not treated with antibiotic therapy(28).
The healing rate proven by histopathological examination of chronic endometritis was 73% using a combination of ofloxacin (400 mg, twice daily, for 14 days) and metronidazole (500 mg, twice daily, for 14 days)(27).
Oral antibiotic therapy, associated with oral and vaginal probiotics, plays an important role in regulating the endometrial microbiome. Oral antibiotic treatment destroys a vast part of the endometrial microbiome, and probiotics contribute to the restoration of lactobacilli that maintain a normal balance in the endometrial cavity(9,14,17). In the study published by Kyono et al., the recovery of endometrial microbiota following a combination of oral probiotic and vaginal probiotic treatment was faster than in the case of single treatment with vaginal probiotics(14).
Conclusions
In cases of recurrent implantation failures, in the absence of other tumors or malformation causes, it is recommended to verify the endometrial microbiome. Hysteroscopy performed before embryo transfer should involve an endometrium biopsy, especially if the uterine cavity suggests chronic endometritis(15,29).
The treatment of endometritis or dysbiosis is associated with an increase in the implantation rate and live births.
Conflict of interest: none declared
Financial support: none declared
This work is permanently accessible online free of charge and published under the CC-BY.

Bibliografie
- Cicinelli E, De Ziegler D, Nicoletti R, et al. Chronic endometritis: correlation among hysteroscopic, histologic, and bacteriologic findings in a prospective trial with 2190 consecutive office hysteroscopies. Fertil Steril. 2008;89(3):677-684.
- Kamiyama S, Teruya Y, Nohara M, Kanazawa K. Impact of detection of bacterial endotoxin in menstrual effluent on the pregnancy rate in in vitro fertilization and embryo transfer. Fertil Steril. 2004;82(4):788-792.
- Liu Y, Chen X, Huang J, et al. Comparison of the prevalence of chronic endometritis as determined by means of different diagnostic methods in women with and without reproductive failure [published correction appears in Fertil Steril. 2019 Feb;111(2):411]. Fertil Steril. 2018;109(5):832-839.
- Cicinelli E, De Ziegler D, Nicoletti R, et al. Poor reliability of vaginal and endocervical cultures for evaluating microbiology of endometrial cavity in women with chronic endometritis. Gynecol Obstet Invest. 2009;68(2):108-115.
- Moreno I, Cicinelli E, Garcia-Grau I, et al. The diagnosis of chronic endometritis in infertile asymptomatic women: a comparative study of histology, microbial cultures, hysteroscopy, and molecular microbiology. Am J Obstet Gynecol. 2018;218(6):602.e1-602.e16.
- Park HJ, Kim YS, Yoon TK, Lee WS. Chronic endometritis and infertility. Clin Exp Reprod Med. 2016;43(4):185-192.
- Johnston-MacAnanny EB, Hartnett J, Engmann LL, Nulsen JC, Sanders MM, Benadiva CA. Chronic endometritis is a frequent finding in women with recurrent implantation failure after in vitro fertilization. Fertil Steril. 2010;93(2):437-441.
- Romero R, Espinoza J, Mazor M. Can endometrial infection/inflammation explain implantation failure, spontaneous abortion, and preterm birth after in vitro fertilization?. Fertil Steril. 2004;82(4):799-804.
- Zolghadri J, Momtahan M, Aminian K, Ghaffarpasand F, Tavana Z. The value of hysteroscopy in diagnosis of chronic endometritis in patients with unexplained recurrent spontaneous abortion. Eur J Obstet Gynecol Reprod Biol. 2011;155(2):217-220.
- Văduva CC, Săndulescu MS, Tenovici M, Siminel MA, Novac MB. Results of in vitro fertilization after diagnosis and treatment of chronic endometritis. Eur Rev Med Pharmacol Sci. 2023;27(3):1069-1076.
- Moreno I, Codoñer FM, Vilella F, et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am J Obstet Gynecol. 2016;215(6):684-703.
- Lozano FM, Lledó B, Morales R, Cascales A, Hortal M, Bernabeu A, Bernabeu R. Characterization of the Endometrial Microbiome in Patients with Recurrent Implantation Failure. Microorganisms. 2023;11(3):741.
- Singer M, Borg M, Ouburg S, Morré SA. The relation of the vaginal microbiota to early pregnancy development during in vitro fertilization treatment – A meta-analysis. J Gynecol Obstet Hum Reprod. 2019;48(4):223-229.
- Kyono K, Hashimoto T, Kikuchi S, Nagai Y, Sakuraba Y. A pilot study and case reports on endometrial microbiota and pregnancy outcome: An analysis using 16S rRNA gene sequencing among IVF patients, and trial therapeutic intervention for dysbiotic endometrium. Reprod Med Biol. 2018;18(1):72-82.
- Lédée N, Petitbarat M, Chevrier L, et al. The Uterine Immune Profile May Help Women With Repeated Unexplained Embryo Implantation Failure After In Vitro Fertilization. Am J Reprod Immunol. 2016;75(3):388-401.
- Nwabuobi C, Arlier S, Schatz F, Guzeloglu-Kayisli O, Lockwood CJ, Kayisli UA. hCG: Biological Functions and Clinical Applications. Int J Mol Sci. 2017;18(10):2037.
- Kane N, Kelly R, Saunders PT, Critchley HO. Proliferation of uterine natural killer cells is induced by human chorionic gonadotropin and mediated via the mannose receptor. Endocrinology. 2009;150(6):2882-2888.
- Makrigiannakis A, Vrekoussis T, Zoumakis E, Kalantaridou SN, Jeschke U. The Role of HCG in Implantation: A Mini-Review of Molecular and Clinical Evidence. Int J Mol Sci. 2017;18(6):1305.
- Văduva CC, Constantinescu C, Şerbănescu M, Dîră L, Oancea MD, Carp-Velişcu A. The association between endometrial polyps, chronic endometritis, and IVF outcomes. Eur Rev Med Pharmacol Sci. 2023;27(18):8895-8904.
- Squillace ALA, Simonian DS, Allegro MC, Borges E Júnior, Bianchi PHM, Bibancos M. Adenomyosis and in vitro fertilization impacts – A literature review. JBRA Assist Reprod. 2021;25(2):303-309.
- D’Arpe S, Franceschetti S, Caccetta J, Pietrangeli D, Muzii L, Panici PB. Management of hydrosalpinx before IVF: a literature review. J Obstet Gynaecol. 2015;35(6):547-550.
- Guo XC, Segars JH. The impact and management of fibroids for fertility: an evidence-based approach. Obstet Gynecol Clin North Am. 2012;39(4):521-533.
- Cicinelli E, Resta L, Nicoletti R, Zappimbulso V, Tartagni M, Saliani N. Endometrial micropolyps at fluid hysteroscopy suggest the existence of chronic endometritis. Hum Reprod. 2005;20(5):1386-1389.
- Kitaya K. Prevalence of chronic endometritis in recurrent miscarriages. Fertil Steril. 2011;95(3):1156-1158.
- Bouet PE, El Hachem H, Monceau E, Gariépy G, Kadoch IJ, Sylvestre C. Chronic endometritis in women with recurrent pregnancy loss and recurrent implantation failure: prevalence and role of office hysteroscopy and immunohistochemistry in diagnosis. Fertil Steril. 2016;105(1):106-110.
- McQueen DB, Maniar KP, Hutchinson A, Confino R, Bernardi L, Pavone ME. Redefining chronic endometritis: the importance of endometrial stromal changes. Fertil Steril. 2021;116(3):855-861.
- Cicinelli E, Matteo M, Tinelli R, et al. Prevalence of chronic endometritis in repeated unexplained implantation failure and the IVF success rate after antibiotic therapy. Hum Reprod. 2015;30(2):323-330.
- McQueen DB, Bernardi LA, Stephenson MD. Chronic endometritis in women with recurrent early pregnancy loss and/or fetal demise. Fertil Steril. 2014;101(4):1026-1030.
- Gu J, Sun Q, Qi Y, Hu F, Cao Y. The effect of chronic endometritis and treatment on patients with unexplained infertility. BMC Womens Health. 2023;23(1):345.