Introduction. Genital infections, in both men and women, may cause permanent functional damage to the reproductive tract, resulting in infertility. In men, post-infection infertility is uncommon, whereas in women sequelae after pelvic inflammatory disease (PID) are the most common cause of acquired infertility. The objective was to assess if laparoscopy can improve the outcome in term of infertility in patients with pelvic inflammatory disease. Materials and method. We conducted a descriptive retrospective cohort study in our clinic (Bucur Maternity from Bucharest) between 2016 and 2018 among patients who were admitted for PID. Patient’s age, parity, the anatomoclinical form of the disease, the presence of infertility before and after treatment, the type of surgery and the outcome were assessed. Results. 267 patients with PID were admitted in the three years of the study. Forty-three underwent surgery, of which 18 were laparoscopic. Five patients (28%) suffered from primary or secondary infertility prior to surgery. Almost half of the laparoscopies were performed for unilateral or bilateral sactosalpinx; there were two cases of both endometriosis and PID, two cases of adnexal tumors, one case of bilateral pyosalpinx, and one case of haematosalpinx. The evolution was uneventful in all cases. The patients were followed for a mean period of 12 months after surgery, and hysterosalpingography was performed in order to assess tubal patency. We encountered tubal obstruction in 60% of the cases where tubes were preserved. Conclusions. Minimally invasive surgery is justified in order to diagnose, treat and prevent further fertility implications in patients with PID.
Tratamentul laparoscopic al bolilor pelvine inflamatorii şi implicaţiile infertilităţii
Laparoscopic treatment of pelvic inflammatory disease and infertility implications
First published: 21 iunie 2019
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/ObsGin.67.2.2019.2417
Abstract
Rezumat
Introducere. Infecţiile genitale, atât la bărbaţi, cât şi la femei, pot provoca leziuni funcţionale permanente ale aparatului reproductiv, care pot duce la apariţia infertilităţii. La bărbaţi, infertilitatea post-infecţie este rară, în timp ce la femei, sechelele după boala inflamatorie pelviană (BIP) sunt cea mai comună cauză a infertilităţii dobândite. Obiectivul acestui studiu a fost de a evalua dacă laparoscopia poate îmbunătăţi prognosticul din punctul de vedere al fertilităţii la pacientele cu boală inflamatorie pelviană. Materiale şi metodă. Am efectuat un studiu descriptiv-retrospectiv de tip cohortă în clinica noastră (Maternitatea Bucur din Bucureşti) între 2016 şi 2018, în rândul pacientelor care au fost internate cu BIP. Au fost evaluate vârsta pacientului, paritatea, forma anatomoclinică a bolii, prezenţa infertilităţii înainte şi după tratament, tipul de intervenţie chirurgicală şi rezultatul postoperatoriu. Rezultate. Un număr de 267 de paciente cu BIP au fost internate în cei trei ani evaluaţi. Patruzeci şi trei au suferit o intervenţie chirurgicală, din care 18 au fost laparoscopice. Cinci paciente (28%) au suferit de infertilitate primară sau secundară înainte de operaţie. Aproape jumătate din laparoscopii au fost efectuate pentru sactosalpinx unilateral sau bilateral; au existat două cazuri de endometrioză şi BIP, două cazuri de tumori anexiale, un caz de piosalpinx bilateral şi un caz de hematosalpinx. Evoluţia a fost favorabilă în toate cazurile. Pacientele au fost urmărite pentru o perioadă medie de 12 luni după intervenţia chirurgicală şi a fost efectuată histerosalpingografia pentru a evalua permeabilitatea tubară. Am întâlnit obstrucţia tubară în 60% din cazurile în care trompa a fost păstrată. Concluzii. Intervenţia chirurgicală minim invazivă este justificată pentru diagnosticarea, tratarea şi prevenirea complicaţiilor ulterioare ale fertilităţii la pacientele cu BIP.
Introduction
Pelvic inflammatory disease (PID), a common ailment of the reproductive tract, is diagnosed in more than one million women each year in the United States of America, being one of the most misdiagnosed and inadequately treated illnesses affecting women worldwide. PID is determined by the ascent of bacteria originating from the vagina and cervix upward into the uterus, ovaries, or fallopian tubes. It can lead to an abscess formation in the fallopian tube or ovary. The diagnosis is based on clinical findings in any sexual active woman who present hipogastric abdominal pain, excluding the many other causes that may lead to lower abdominal pain in women(1). PID is usually treated with antibiotics against pathogens such as Neisseria gonorrhoea, Chlamydia trachomatis, fastidious bacterial vaginitis associated bacteria (Sneathia spp., Atopobium vaginae), Ureaplasma or respiratory pathogens (Haemophilus influenza, Streptococcus pneumonia, Staphylococcus aureus) and enteric pathogens (Escherichia coli, Bacteroides fragilis, group B Streptococci) in 15% of cases(2).
Materials and method
We realized a descriptive retrospective study in our clinic, Bucur Maternity from Bucharest, between 2016 and 2018. The inclusion criteria were: patients presenting for chronic pelvic pain or infertility due to PID who underwent laparoscopic exploration. Patients’ age, parity, the anatomoclinical form of the disease, the presence of infertility before and after treatment, the type of surgery, and the outcome were registered.
Results
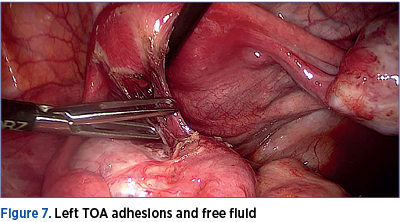
A number of 267 patients with PID were admitted in the three years of the study. The mean age of the patients was 29.3 years old (range: 18-42 years old). Surgery was performed for 43 patients, of which 18 were laparoscopic. Five patients (28%) had primary or secondary infertility prior to surgery. 45% of the laparoscopies were performed for unilateral or bilateral sactosalpinx; there were two cases of both endometriosis and PID, two cases of adnexal tumors, one case of bilateral pyosalpinx, and one case of haematosalpinx. There was only one case of infertility with bilateral hidroosalpinx converted in open surgery due to epigastric inferior artery injury. The evolution was uneventfull in all cases.
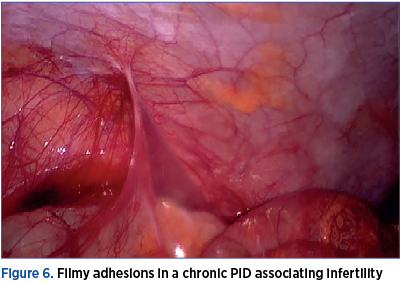
The pathological examination confirmed the laparoscopic diagnosis in 95.5% of the cases with salpingitis. There were identified three cases with Fitz-Hugh-Curtis syndrome. The severity of the cases was not correlated with laparoscopic findings. In some cases with severe pelvic adhesions, the patients had no pelvic pain and the only complain was infertility. One patient underwent two laparoscopies for PID in 12 months due to chronic pelvic pain. The first intervention was conservative therapy with adhesion removal, and during the second one the tubes were removed and the clinical presentation improved. The patients were followed-up for a mean period of 12 months after surgery, and hysterosalpingography was performed in order to assess tubal patency. We encountered tubal obstruction in 60% of the cases where tubes were preserved.
Discussion
In 2005, the World Health Organization (WHO) estimated that approximately 448 million new cases of curable PID occur annually in individuals aged 15-49 years old. Worldwide, WHO has determined that STIs (sexually transmitted infections) rank in the top 5 disease categories for which adults seek care. Women in poor countries, especially those in sub-Saharan Africa and Southeast Asia, experience an increased rate of complications and sequelae. In high-income countries, the annual rate is the highest and it reaches 10-20 per 1000 women of reproductive age. Public health efforts implemented in Scandinavia to decrease the prevalence of STIs have been quite effective in reducing the incidence of PID(3).
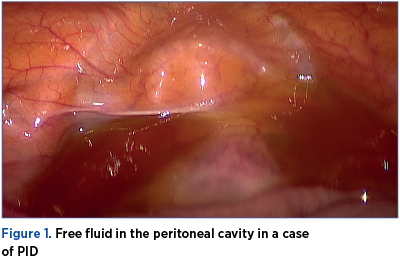

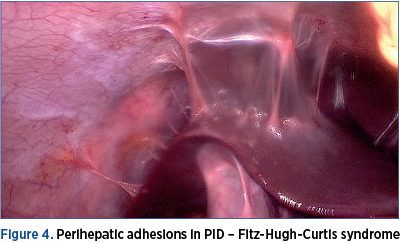
In human studies, the evaluation of tubal inflammation is difficult without surgical intervention; thus, many studies use endometritis as a marker for tubal inflammation, making endometrial biopsies a useful tool in diagnosing PID. Tubal damage is best described in the context of chlamydial infection and appears to be related both to an innate immune inflammatory response initiated by the epithelial cells infected by C. trachomatis, and to an adaptive T-cell response(4). Key components of the physical exam include: abdominal exam, including palpation of the right upper quadrant (Fitz-Hugh-Curtis syndrome), vaginal speculum exam, checking for friability and leucorrhoea, bimanual exam, assessing for cervical motion, uterine, or adnexal tenderness, as well as pelvic masses and microscopic evaluation of a sample of cervicovaginal discharge to assess for T. vaginalis, bacterial vaginosis and/or leukorrhea.
Although the presenting signs and symptoms of a tubo-ovarian abscess (TOA) are similar with those of salpingitis/endometritis, there are often more objective signs of infection and inflammation. A large series of patients with ultrasound-confirmed or surgically-confirmed TOA found that 60% of them had a temperature >37.8°C, 68% had a leucocytosis >10,000 cells/mL, 26% had nausea, and 19% had chronic abdomino-pelvic pain. In women with PID, the palpation of an adnexal mass on the physical exam, significant pain limiting proper evaluation of the adnexa, severe illness, or lack of clinical response to antimicrobial therapy should prompt imaging studies. Additionally, imaging can be helpful to evaluate for alternative diagnoses such as appendicitis, ovarian torsion or cyst rupture(5).
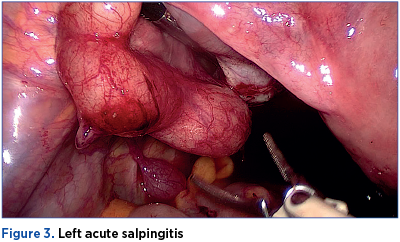
Among hemodynamically stable women with a tubo-ovarian abscess, observation is recommended for at least 24 hours, with the aim of observing for early signs of sepsis or potential abscess rupture. The surgical exploration on initial evaluation is indicated in the setting of an acute abdomen and signs of sepsis or hemodynamic instability, particularly if a ruptured TOA is suspected. Antimicrobial therapy should always start parenteral, and should cover anaerobes (clindamycin or metronidazole). Antimicrobial therapy alone, with appropriate anaerobic coverage and the ability to penetrate and function in abscess cavities, is effective in 70-84% of women(6).
In one cohort of women admitted with TOA, 60% of those with an abscess larger than 10 cm needed surgical management, compared to 20% of those with 4-6 cm abscesses. When no clinical improvement is noted within 72 hours of antibiotic initiation, minimally invasive drainage of the abscess or surgical management can be pursued; however, significant clinical deterioration at any time point usually indicates the need for surgical exploration. A study of empirical transvaginal ultrasound-guided aspiration of TOAs at the time of diagnosis, in association with antimicrobial therapy, revealed that the procedure was safe, well tolerated, and avoided surgical management in 93% of cases. Although rare, post-menopausal women can develop PID, presenting most commonly with hypogastric pain and postmenopausal bleeding, as well as fever, nausea and altered bowel habits. Those women are considerably more likely to develop TOAs(7). In several small case series, the pathology of the surgical specimens revealed a concurrent gynaecologic malignancy (cervix, endometrium, or ovary) in 40-47% of the patients. Based on these data, any postmenopausal woman with PID should be evaluated for the presence of a pelvic cancer(8).

A review of studies assessing PID after IUD insertion in the presence of Neisseria or chlamydial cervicitis showed an increased but overall quite low risk (0-5%). Recent studies suggest that screening for this germs at the time of insertion, as opposed to requiring a negative test prior to the procedure, does not significantly increase adverse sequelae. There seems to be no difference in PID risk for the hormone releasing DIU compared with copper IUDs. The presence of an intrauterine device (IUD) at the time of diagnosis of acute PID does not alter the management, and empiric removal of the IUD is not indicated(9).
The differential diagnosis for PID includes gynaecologic diseases or dysfunction (complications of pregnancy, acute infections, endometriosis, adnexal disorders like ovarian torsion and ovarian cyst rupture, menstrual disorders, ectopic pregnancy), as well as gastrointestinal (appendicitis, gastroenteritis, inflammatory bowel disease), genitourinary (cystitis, pyelonephritis, nephrolithiasis), musculoskeletal and neurologic causes, and also traumatic injury(10). The gold standard diagnosis technique for PID is laparoscopy. It represents the only method that can describe the macroscopic aspect of the tubes and establishes the diagnosis of salpingitis(11).
The first reports about PID and laparoscopy were based on 905 cases with chronic pelvic pain. Then, there were also described the standard elements for laparoscopic findings. These include: oedema of the tubal wall, extensive hyperaemia of the tubal surface or exudate on the tubal surface. In this study, out of 814 cases with suspected acute PID, 532 (65%) had acute salpingitis confirmed during laparoscopy, 12% had pain caused by other conditions, and 23% had no pathology.
There were compared the clinical, laboratory and laparoscopic findings for acute PID. The most important remark was the lack of correlation between clinical, laboratory and laparoscopic findings. The severity of the clinical symptoms was not associated with tubal occlusion. Even though laparoscopy is considered the gold standard for PID, the reviews from literature regarding its accuracy are controversial(12). The accuracy of clinical diagnosis with laparoscopic diagnosis for PID has been reported in different studies. In a prospective case-control study, there was investigated the aetiology of salpingitis for 158 women with a clinical diagnosis of acute PID, and salpingitis was confirmed by laparoscopy in 142 cases (90%). Other authors described a low correlation between the clinical appearance of PID and laparoscopic observation of fallopian tubes with oedema, erythema and purulent exudates. In a recent study, the sensitivities of laparoscopic triad of oedema, erythema and purulent exudates for PID clinical criteria was 65%, while the specificity was 60%(13). In another study, the authors found the sensitivity of laparoscopy for PID between 50% and 80%. Further information were offered by endometrial biopsy that increased the prevalence of PID from 30% in visual diagnosis to 46% when endometrial mini-biopsy evidence was included(14).
Unfortunately, the impact of infectious sequelae on human reproduction continues to increase as a consequence of sexual promiscuity, the lack of sexual education, and the popularity of non-barrier methods of contraception. C. trachomatis and gonorrhoeal infections, as well as mixed anaerobic infections are the most prevalent causes of upper genital tract infections resulting in pelvic inflammatory disease. Bacterial vaginitis, Trichomonas vaginalis and Candida albicans are the most frequent bacterial, protozoan and fungal causes of lower genital tract infections. Although gonorrhoeal infections have been on the decline in the last decade, chlamydial infections of the male and female genital tract continue to be an increasing problem, and C. trachomatis is the major cause of tubal factor infertility(15).
Conclusions
PID continues to be a burden for health services, considering the wide spread in women of reproductive ages. Its consequences on further fertility is of great impact, being one of the most frequent causes for tubal occlusion.
The majority of PID forms can benefit from minimally invasive surgery as a diagnostic tool. Laparoscopy can also treat some forms as TOA, it’s useful for adhesiolysis and prevents further fertility complications in patients with PID.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
2. Miron ND, Socolov D, Mareş M, et al. Bacteriological agents which play a role in the development of infertility. Acta Microbiol Immunol Hung. 2013 Mar; 60(1):41-53.
3. Moore Shepherd S. What is the global incidence of pelvic inflammatory disease (PID)? Available from https://www.medscape.com/answers/256448-25900/what-is-the-global-incidence-of-pelvic-inflammatory-disease-pid
4. The American College of Obstetricians and Gynecologists. Pelvic Inflammatory Disease. Available from https://www.acog.org/Patients/FAQs/Pelvic-Inflammatory-Disease-PID?IsMobileSet=false
5. Haggerty CL, Totten PA, Tang G, et al. Identification of novel microbes associated with pelvic inflammatory disease and infertility. Sex Transm Infect. 2016; 92:441-446.
6. Schuchardt L, Rupp J. Chlamydia Trachomatis as the Cause of Infectious Infertility: Acute, Repetitive or Persistent Long-Term Infection?. In: Häcker G. (eds) Biology of Chlamydia. Current Topics in Microbiology and Immunology. 2016; vol 412. Springer, Cham.
7. Jaiyeoba O, Soper DE. A Practical Approach to the Diagnosis of Pelvic Inflammatory Disease. Infectious Diseases in Obstetrics and Gynecology. 2011; vol. 2011, article ID 753037.
8. Sweet RL. Treatment of acute pelvic inflammatory disease. Infect Dis Obstet Gynecol. 2011; 561909.
9. Price MJ, Ades AE, Welton NJ, Simms I, Macleod J, Horner PJ. Proportion of Pelvic Inflammatory Disease Cases Caused by Chlamydia Trachomatis: Consistent Picture From Different Methods. J Infect Dis. 2016; 214(4):617–624.
10. Jennings LK, Krywko DM. Pelvic Inflammatory Disease (PID). Available from: https://www.ncbi.nlm.nih.gov/books/NBK499959/
11. Pleş L, Sima RM, Burnei A, et al. The experience of our Clinic in laparoscopy for adnexal masses and the correlation between ultrasound findings and pathological results. Rom J Morphol Embryol. 2016; 57(4):1337–1341.
12. Tai FW, Chang CY, Chiang JH, Lin WC, Wan L. Association of Pelvic Inflammatory Disease with Risk of Endometriosis: A Nationwide Cohort Study Involving 141,460 Individuals. J Clin Med. 2018; 7(11):379. Published 2018 Oct 24.
13. Mitchell C, Prabhu M. Pelvic inflammatory disease: current concepts in pathogenesis, diagnosis and treatment. Infect Dis Clin North Am. 2013; 27(4):793–809.
14. Pleş L, Sima RM, Moisei C, Ionescu CA. An intrauterine contraceptive device: where did we find it after 29 years of insertion? A case report. J Pak Med Assoc. 2017 Jan; 67(1):131-133.
15. Dalal R. Infection and Infertility. Available from: https://www.intechopen.com/books/genital-infections-and-infertility/infection-and-infertility
Articole din ediţiile anterioare
Rolul chirurgiei în cazul pacientelor infertile cu adenomioză
Adenomioza este o maladie benignă ce aparţine unui grup de afecţiuni estrogen-dependente, asociate cu unele consecinţe clinice semnificative: disme...
Sensibilitatea şi specificitatea 3D/4D HyCoSy în evaluarea permeabilităţii tubare în context clinic
Scop. Obiectivul acestui studiu este de a evalua performanţa histerosonosalpingografiei de contrast 3D/4D (HyCoSy) în evaluarea permeabilităţii tu...
Managementul histeroscopic al subfertilităţii în cazurile cu suspiciune de polipi endometriali
Polipii endometriali reprezintă o afecţiune ginecologică benignă asociată cu sângerări uterine anormale, infertilitate şi pierderi repetate de sarc...
Tipare de infertilitate. Un studiu de asistenţă medicală primară
Introducere. Infertilitatea reprezintă o problemă medicală şi economică majoră în întreaga lume. Există puţine date referitoare la tiparele cauzelo...