Pleural malignant mesothelioma is an aggressive form of malignancy, typically related to asbestos exposure and occurring several decades after exposure. We present the case of a 73-year-old male patient, with no apparent exposure to asbestos, non-smoker, in whom a pleural effusion was discovered accidentaly. Pleural needle biopsy showed an epithelioid mesothelioma, confirmed by immunostains. PET-CT scan confirmed the pleural extension of the tumor. The non-asbestos causes of mesothelioma are discussed.
Mezoteliom malign pleural la un pacient fără expunere la azbest
Pleural malignant mesothelioma in a patient with no asbestos exposure
First published: 30 martie 2020
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/OnHe.50.1.2020.2961
Abstract
Rezumat
Mezoteliomul malign pleural reprezintă o formă agresivă de malignitate, de obicei legată de expunerea la azbest şi apărând la câteva decenii după expunere. Prezentăm cazul unui bărbat de 73 de ani, fără expunere aparentă la azbest, nefumător, la care un revărsat pleural a fost descoperit accidental. Biopsia pleurală a arătat un mezoteliom epitelioid, confirmat de imunohistochimie. Scanarea PET-CT a confirmat extensia pleurală a tumorii. Sunt discutate cauzele non-azbest ale mezoteliomului.
Introduction
Malignant pleural mesothelioma is an aggressive tumor with unfavourable prognosis. The main factor typically involved in the pathogenesis of the disease is asbestos fibers exposure, the occupational exposures being present in most cases. The latency of the disease is about 40 years, and the development of the disease is not dose-dependent. Most patients present with thoracic or respiratory symptoms, like diffuse persistent chest pain or dyspnea. Very few patients are asymptomatic at the moment of diagnosis.
We present the case of a 73-year-old male patient, with no respiratory symptoms, investigated for digestive complaints and with a pleural effusion discovered accidentally. The consequent investigations showed a malignant pleural mesothelioma.
Case presentation
Patient D.A., 73 years old, male, never smoker, with no history of occupational or domestic exposure to allergens, dusts or fibers, presented in November 2019 in a gastroenterology department for digestive symptoms consisting of alternance of constipation and diarrhea. The medical history consisted of repeated episodes of atrial fibrilation and mild mitral insuficiency, on treatment with oral anticoagulants of new generation (NOAC), and colonic polyposis diagnosis a year before by colonoscopy.
Blood tests showed no particular changes, except for a mild increase of erithrocyte sedimentation rate (35 mm in 1 hour) and mild decrease of iron level. Chest X-ray performed on this occasion showed a minimal opacifiation of the left costo-frenic angle. A gastric endoscopy was performed, showing erosive gastritis. An abdominal computed tomography (CT) was performed, with no particular abdominal findings, but the upper abdomen slices showed the presence of pleural fluid in the left pleura. The patient was referred to the “Marius Nasta” Institute of Pneumophthisiology for further pulmonary check-up.
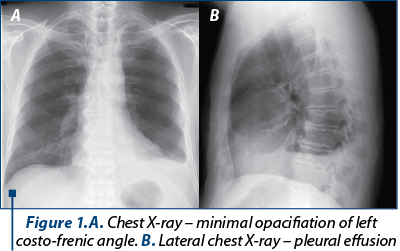
The physical examination showed a patient in good state, pale, with moderate dullness at the base of left hemithorax and diminished lung sounds, with an oxygen saturation rate of 96% at rest in the ambient air, and no other abnormal findings.
The chest X-ray showed opacifiation of the left costo-frenic angle, more obvoius than the previous chest X-ray, with visible pleural effusion on the lateral view (Figure 1). The electrocardiogram was normal.
A thoracocentesis was performed, extracting 15 mL of fluid with moderate bloody aspect. The biochemistry showed an exsudate with 6.2 g/dL proteins, but normal LDH (170 UI/L), and low adenosin-desaminase (ADA) (24 UI/L). The cytologic examination of the fluid showed a high percentage of lymphocytes (85%) and no malignant cells.
After stopping the oral anticoagulant therapy for five days, a percutaneous pleural biopsy was performed, using the Stoicescu-Bercea pleural biopsy needle. Four pleural fragments were taken, and a new sample of pleural fluid for cytology and biochemistry examinations. These reconfirmed the initial findings, of exsudate with proteins 3.9 g/dL, ADA 33 U/L, LDH 288 U/L, and a lymphocytic predominance (lymphocytes 90%) with no malignant cells. The histological examination of the pleural tissue showed epithelioid cell infiltrates with trabecular disposition, with the diagnosis of diffuse epithelioid malignant mesothelioma (Figure 2).
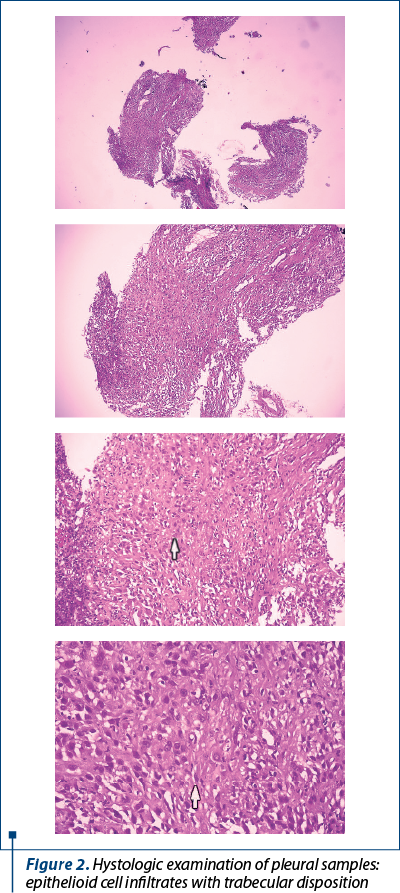
The immunostains performed showed the following phenotype: strong and diffuze positivity for Calretinin (nuclear and cytoplasmic) and Podoplanin/D2-40 (membrane) and negativity for TTF1, CK7, CK BerEP4, in the tumor cells ; the nuclear index expression/Ki 67 was 24% in tumor mesothelial cells; with the conclusion of pleural epithelioid malignant mesothelioma diagnostic.
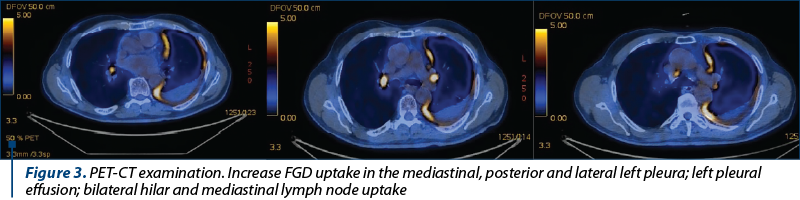
For a better evaluation of the disease extension, the patient chose to have a positron-emission tomography scan (PET-CT). This showed a diffuse extension of the malignancy with intense uptake in the left pleura, involving especially mediastinal pleura and at a lesser extent the posterior and lateral pleura, no uptake in the pleural fluid and bilateral mediastinal lymph node involvement (Figure 3). No FDG uptake was noticed in the abdomen.
The patient was referred to the oncology department for oncologic treatment.
Discussion
Malignant pleural mesothelioma is a rare malignancy, very aggresive and with an unfavourable prognosis, accounting for about 5% of malignancies involving the pleura(1). The exposure to asbestos fibres is the main risk factor involved in the pathogenesis of the disease. Occupational exposure to asbestos can be identified in 70-90% of cases(2). The long duration of latency, of 30 to 40 years after exposure, makes early diagnosis very difficult(1), most patients being diagnosed in advanced stages of the disease(3). The clinical manifestations are nonspecific. Thoracic pain, diffuse and persistent, having only rarely pleuretic features, can sometimes be the only clinical manifestation(2), or it can be accompanied by dyspnea and cough, typically dry. Very few patients are diagnosed accidentally, complaining of no symptoms(4).
The most frequent imaging changes on the plain chest X-ray are pleural effusion or pleural thickening(1). Thoracic CT scan allows a better characterisation of the imaging changes, showing the typical nodular thickening of the pleura or pleural masses. Other changes on CT are reduction of lung volume, traction on the mediastinal structures and pleural calcification(5).
Magnetic resonance imaging (MRI) is not routinely used for investigating malignant mesothelioma, but it can be useful for evaluating the invasion in the thoracic wall, mediastinum or diaphragm. PET-CT or PET 18F-FDG examination has a grat accuracy for differentiating malignant from benign lesions of the pleura and allows the evaluation of local extension, metastasis and the treatment follow-up(6).
A pleural biopsy obtained by thoracoscopy leads to a positive diagnosis in over 90% of cases, allowing the visual exploration of the pleural cavity and to perform biopsies from the most relevant lesions and obtaining enough tissue(1). Histopathologic and immunostains examination of the tumor tissue establishes the final diagnosis, with three histologic types of diffuse mesothelioma being described: epithelioid, sarcomatoid and biphasic. Sometimes, making the difference between malignant mesothelioma and the benign pleural reactive mesothelial lesions or secondary pleural adenocarcinoma can be difficult, even with the use of immunostains(1,7).
Our patient was initially evaluated for digestive symptoms, apparently not associated with the disease. The abdominal CT scan failed to prove any abdominal changes, but identified an asymptomatic pleural effusion. In subsequent pulmonology check-up, the patient didn’t complain of any respiratory symptoms, but the physical examination confirmed the presence of a left pleural effusion. The thoracentesis extracted a small quantity of pleural fluid presenting no malignant cells, but a high percentage of lymphocytes. In an asymptomatic elderly patient in a country with high incidence of tuberculosis, the possibility of a tuberculosis pleural effusion was taken into account, based on the high lymphocyte count in the pleural fluid, and a percutaneous needle pleural biopsy was performed. The diagnosis yield of needle biopsy is over 60% for tuberculosis, and this technique was prefered as initial diagnostic approach as it is well tolerated and with minimal risks by using the Stoicescu-Bercea pleural biopsy needle. The pathologic examination of the tissue samples proved the diagnosis of diffuse epithelioid malignant mesothelioma. Thus, the tissue samples taken by the simple needle biopsy were enough for establishing the diagnosis, and the pathologic examination was highly suggestive for mesothelioma even before the immunohistochemistry confirmation. At this stage, further examination by thoracoscopy was not considered necessary.
The PET-CT scan performed afterwards allowed the characterisation of the pleural malignancy, describing important left pleural extension, with involvement of mediastinal, lateral and posterior pleura, and the evaluation of lymph node involvement. No remote metastases or invasion of thoracic wall, mediastinum or diaphragm were noticed.
The medical and exposure history was repeated, but no asbestos exposure could be identified.
Non-asbestos etiology of mesothelioma is very diverse and needs to be checked in patients with mesothelioma without any obvious asbestos exposure. Non-asbestos mineral fibres like erionite or fluoro-edenite can induce the development of mesothelioma, being identified as etiologic agents in patients from regions where such mineral deposits are abundant(8). In Anatolia, Turkey, erionite is the main cause of mesothelioma, the exposure being identified in more than 50% of patients(9,10).
Ionizing radiation is well recognized as carcinogenic inducer. Published data suggest the association between exposure to ionizing radiation and the development of mesothelioma(8). Cases of mesothelioma (pleural, peritoneal or percardial) were described in patients receiving radiotherapy for various malignancies, like Hodgkin or non-Hodgkin lymphomas, breast cancer and Wilms tumor(8,11-13). Occupational exposure to gamma radiation also seems to be associated to an increased risk for mesothelioma(14).
Chronic pleural inflammation, present in patients with long-standing pleural empyema, is anectodically associated to the development of malignant mesothelioma, as showen by several case reports, but supported by little data(8). The involvement of the simian virus 40 (SV40) in the etiology of mesothelioma was studied, based on the tumor-like changes induced by the intrapleural or intracardiac injection of the virus in laboratory animals(15). Still, this does not seem to be an etiologic agent for mesothelioma in humans(8). Epidemiologic studies failed to prove an association between the use of anti-polio vaccine contaminated with SV40 (in the 1950-1960 period) and the incidence of mesothelioma(4,16,17).
It is estimated that less than 1% of all mesothelioma cases can be caused by a mutation of the gene coding BAP-1 protein (BRCA1-associated protein-1)(18).
All these non-asbestos etiologies are associated to the development of mesothelioma in a minority of patients. Most cases of mesothelioma not clearly associated to asbestos exposure are considered spontaneous (idiopathic)(8). This is most probably also the case for our patient. We cannot rule out an occult asbestos exposure in an elderly patient, which was possible 40 years ago, in a time when the asbestos was still present in all kind of industrial and construction materials and in vehicles’ breaking systems, before asbestos was excluded from these materials.
Prognosis and staging
The most used pleural mesothelioma staging was proposed by IMIG (International Mesothelioma Interest Group), based on TNM classification(4). Negative prognosis factors are transdiaphragmatic invasion, involvement of mediastinal lymph nodes, male sex, age over 75 years old, thoracic pain, reduced performance status, leukocytosis, thrombocytosis, and non-epithelioid type(1,4). In our case, the patient is included in stage III according to TNM classification, given the absence of extension outside left pleural cavity, and involvement of hilar and mediastinal lymph nodes. The prognosis is unfavourable, despite the epithelioid hystologic type and absence (for the moment) of thoracic pain.
Particular features of the case
Accidental discovery of a malignant pleural mesothelioma in a patient with no respiratory symptoms (chest pain, dyspnea, cough), and in the absence of asbestos exposure.
Conflicts of interests: The authors declare no conflict of interests.
Bibliografie
- Scherpereel A, Astoul P, Baas P, et al. Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J. 2010; 35:479–95.
- Mott FE. Mesothelioma: a review. The Ochsner Journal. 2012; vol. 12,1: 70-9.
- Flores RM, Zakowski M, Venkatraman E, et al. Prognostic factors in the treatment of malignant pleural mesothelioma at a large tertiary referral center. J Thor Oncol. 2007; 2:957–965.
- Chapman S, Robinson G, Stradling J, West S, Wrightson J. Oxford Handbook of Respiratory Medicine, 3 ed., Oxford University Press, 2014, chapter 17, pg. 118-121
- Klawiter A, Damaszke T. Pleural mesothelioma – case report. Pol J Radiol. 2010; 75(4): 61-63.
- Bonomi M, De Filippis C, Lopci E, Gianoncelli L, Rizzardi G, Cerchiaro E, Bortolotti L, Zanello A, Ceresoli GL. Clinical staging of malignant pleural mesothelioma: current perspectives. Lung Cancer (Auckl). 2017; 8:127-139, https://doi.org/10.2147/LCTT.S102113
- Ahmad M, Aamir MO, Minhas K, Ajmal K, Ahmad I. Malignant pleural mesothelioma: Presentation of a case report. Egyptian Journal of Basic and Applied Sciences. 2018; 5, 234–236.
- Jasani B, Gibbs A. Mesothelioma Not Associated With Asbestos Exposure. Archives of Pathology & Laboratory Medicine. March 2012; Vol. 136, No. 3, pp. 262-267.
- Baris YI, Sahin AA, Ozesmi M, et al. An outbreak of pleural mesothelioma and chronic fibrosing pleurisy in the village of Karain/Urgup in Anatolia. Thorax. 1978; 33(2):181–192.
- Artvinli M, Baris YI. Malignant mesotheliomas in a small village in the Anatolian region of Turkey: an epidemiologic study. J Natl Cancer Inst. 1979; 63(1):17–22.
- Austin MB, Fechner RE, Roggli VL. Pleural malignant mesothelioma following Wilms’ tumor. Am J Clin Pathol. 1986; 86(2):227–230.
- Shannon VR, Nesbitt JC, Libshitz HI. Malignant pleural mesothelioma after radiation therapy for breast cancer: a report of two additional patients. Cancer. 1995; 76(3):437–441.
- Teta MJ, Lau E, Sceurman BK, et al. Therapeutic radiation for lymphoma: risk of malignant mesothelioma. Cancer. 2007; 109(7):1432–1438.
- Horie A, Hiraoka K, Yamamoto O, et al. An autopsy case of peritoneal malignant mesothelioma in a radiation technologist. Acta Pathol Jpn. 1990; 40(1):57–62.
- Cicala C, Pompetti F, Carbone M. SV40 induces mesotheliomas in hamsters. Am J Pathol. 1993; 142(5):1524–1533.
- Strickler HD, Goedert JJ, Devesa SS, Lajey J, Fraumeni JF, Rosenberg PS. Trend in U.S. pleural mesothelioma incidence rates following simian virus 40 contamination of early poliovirus vaccines. J Natl Cancer Inst. 2003; 95(1):38–45.
- Engles EA, Katki HA, Nielsen NM, et al. Cancer incidence in Denmark following exposure to poliovirus vaccine contaminated with simian virus 40. J Natl Cancer Inst. 2003; 95(7):532–539.
- Rusch A, Ziltener G, Nackaerts K, Weder W, Stahel RA, Felley-Bosco E. Prevalence of BRCA-1 associated protein 1 germline mutation in sporadic malignant pleural mesothelioma cases. Lung Cancer. 2015 Jan;87(1):77-9. Epub 2014 Nov 6.