New approaches to colorectal cancer
Noi abordări în cancerul colorectal
Abstract
Colon cancer is one of the most common localizations of cancer in our time. In this short presentation, I will present some aspects of the systemic therapy of advanced colon cancer. Not being able to have an exhaustive approach, I consider that emphasizing the importance of ctDNA determination in the therapeutic indication and the involvement of precision medicine in the treatment of this cancer are of first importance. Also, colon cancer is found especially at advanced ages, and I considered it necessary to emphasizethe role of geriatric consultation in these patients. This review also presents a practical aspect in chemotherapy for the elderly, namely the stability of the indication for cytostatic oxaliplatin treatment which in certain patients does not bring any benefit.
Keywords
colon cancerelderly patientsnew drugsRezumat
Cancerul colonului reprezintă una din cele mai frecvente localizări ale cancerului în epoca noastră. În această scurtă expunere, vom prezenta câteva aspect ale terapiei sistemice a cancerului de colon avansat. Neputând avea o abordare exhaustivă, am consideratcă sublinierea importanţei pe care o are determinarea ctDNA în indicaţia terapeutică şi implicarea medicinei de precizie în tratamentul acestui cancer sunt de primă importantă. De asemenea, deoarece cancerul de colon este întâlnit în special la vârste înaintate, am considerat necesar de a sublinia rolul consultului geriatric la aceşti pacienţi. Review-ul mai prezintă şi un aspect practic în chimioterapia persoanelor în vârstă – şi anume, stabilirea indicaţiei de tratament citostatic cu oxaliplatină, care la anumiţi pacienţi nu aduce niciun beneficiu.
Cuvinte Cheie
cancer de colonpacienţi vârstnicimedicamente noiThe first trial from my presentation is CALGB (Alliance)/SWOG 80405. This was a a phase III randomized trial that enrolled in the first line patients with metastatic colorectal cancer (mCRC) treated with bevacizumab or cetuximab with chemotherapy. The aim was to discover novel mutated genes associated with prognosis and differential response to therapy with the biologics.
The primary DNA tumor was detected. The effect of mutated genes and mutations on overall survival (OS) was tested adjusting for microsatellite instability status, BRAF V600E, all RAS mutations, arm, sex, and age.
The median number (lower-upper quartile) of mutated genes was 5 (3-7), 5 (3-6) in microsatellite stable, and 12.5 (4.5-32) in microsatellite instability-high tumors.
Mutated KRAS and APC were more frequent in Afro-American (53% and 85%) than in Caucasian patients (27% and 65%, respectively), while BRAF V600E was less frequent in Afro-American (5%) than Caucasian patients (14%). The median OS in patients with BRAF non-V600E (2.2% of patients) was 31.9 months (95% CI; 15.1 to not applicable [NA]), similar to that of BRAF wild-type (wt) patients (31.2 months; 95% CI; 29 to 33.9). Mutated LRP1B (10.7% of patients) was associated with improved OS compared with wt LRP1B (hazard ratio 0.57; 95% CI; 0.40 to 0.80). RNF43 (5.6% of patients) interacted with treatment arms, as in the cetuximab arm, patients with mutated RNF43 had a median OS of 11.5 (95% CI; 10.8 to NA) months compared with 30.1 (95% CI; 24.9 to 35.3) months in patients with wt RNF43, whereas in the bevacizumab arm, patients with mutated RNF43 had a median OS of 25 (95% CI; 14.2 to NA) months compared with 31.3 (95% CI; 29 to 34.3) months in patients with wild-type RNF43(1).
Another study
In a prespecified exploratory biomarker analysis of the randomized, open-label, phase III PARADIGM study, the Japanese researchers investigated the potential prognostic and predictive role of hyperselecting patients for anti-EGFR treatment based on detection of a broad array of genetic alterations in plasma circulating tumor DNA (ctDNA) in patients with RAS wild-type (wt) unresectable metastatic colorectal cancer (mCRC)(2).
Initially, this randomized phase III study was originally designed to demonstrate the superiority of panitumumab (PAN) versus bevacizumab (BEV), both in combination with mFOLFOX6, for RAS wild-type mCRC, but the protocol was revised to analyze the efficacy in patients with a left-sided primary tumor as the primary (final) analysis.
DNA analysis for some genes showed the following results:
Among 802 patients in the full analysis set, 733 (91%) had evaluable pretreatment samples for ctDNA analysis. Of these patients, 204 (28%) had at least one gene alteration, including KRAS/NRAS (8%), BRAF V600E (11%), PTEN (5%), EGFR (3%), HER2 (5%), MET (1%), and fusions (1%). In 529 (72%) hyperselected patients without any gene alterations, overall survival tended to be longer with PAN versus BEV regardless of primary sidedness; the overall survival hazard ratios ranged from 0.76 to 0.82, and the median OS gains ranged from 6.6 to 8 months. Meanwhile, the overall survival was similar or inferior with PAN versus BEV, irrespective of the primary sidedness in patients with any of these gene alterations. As a conclusion, negative hyperselection using ctDNA rather than tumor sidedness may identify appropriate patients for first-line PAN over BEV. These results warrant further validation in additional cohorts(3).
An important trial regarding chemotherapy of senior adults could change the clinical practice.
Oxaliplatin in older adults
Professor David Karr presented a study of the QUASAR group. The premise of this study was the fact that colorectal cancer is a disease of the elderly, with the median age of presentation around 72 years old. We know that, at presentation, more than 50% of patients are aged 65 or over, and one-third of patients are 75 or older. The authors ask themselves: are we justified in giving combination chemotherapy with oxaliplatin to high-risk resected colorectal cancer patients? In this respect, it is presented the meta-analysis by Dottorini and colleagues that came out recently in the Journal of Clinical Oncology. “According to the results of their study, it could be concluded that the addition of oxaliplatin to adjuvant therapy for resected high-risk colorectal cancer in older patients (over 70 years old) doesn’t result in any statistically significant gain in terms of preventing recurrences or saving lives”.
Returning to the QUASAR study, the aims of this study was to determine the size and duration of any survival benefit from adjuvant chemotherapy for patients with colorectal cancer at low risk of recurrence, for whom the indication for such treatment is unclear.
The conclusion was that “chemotherapy with fluorouracil and folinic acid could improve survival of patients with stage II colorectal cancer, although the absolute improvements are small: assuming 5-year mortality without chemotherapy is 20%, the relative risk of death seen here translates into an absolute improvement in survival of 3.6% (95% CI; 1-6)”(4,5). Finaly, professor David Karr concluded that the subject remains unclear: “we need more trials of chemotherapy in older folks to see if the addition of drugs like oxaliplatin to a fluoropyrimidine backbone really does make a difference. I’ve said many times before that we, the medical community recommending adjuvant treatment, need to have better risk stratifiers”.
Some researchers recommend COLOXIS to predict the benefit of oxaliplatin for adjuvant chemotherapy in colon cancer. “COLOXIS is a remarkable model that uses machine learning to analyze patient data and determine the potential benefit of oxaliplatin in early colon cancer treatment. The model was tested on 1065 patients from the NSABP C-07 and C-08 studies, which utilized oxaliplatin in the treatment of colon cancer. The results were very promising. COLOXIS successfully dichotomized patients into two categories, signature-positive and signature-negative. This categorization indicates whether a patient would benefit from oxaliplatin or not. Notably, the COLOXIS-positive patients showed a significant advantage from the addition of oxaliplatin in their treatment, while the COLOXIS-negative patients did not”(6).
Particularities of treatment of colorectal cancer in elderly patients
Approximately half of the incidence of colorectal cancer is registered in patients above 70 years of age in Western countries. Elderly CRC patients, however, are understaged, undertreated and underrepresented in clinical trials. Probably, for these patients, real-world studies will give the best information regarding treatment options.
SIOG has a set of recommendations based on discussions between specialists: surgeons, radiation oncologists and medical oncologists. The key areas of discussions were:
1. Diagnosis, staging and patient assessment.
2. Surgical management of the elderly patient.
3. Radiotherapy in rectal cancer in the elderly patient.
4. Chemotherapy and targeted therapies in the elderly CRC patients
a) adjuvant
b) metastatic.
The recommendations for the diagnosis of tumors of the colon or rectum were as follows:
Suspected cancer of the colon and rectum should be confirmed by colonoscopy.
For confirmed cancer of the colon, a CT of the chest and abdomen and an MRI or ultrasound scan of the abdomen plus chest X-ray (CXR) should be undertaken for staging.
For confirmed rectal cancer, an MRI scan of the pelvis and a CT of the chest and abdomen are necessary. MRI or ultrasound scan of the abdomen plus CXR should be undertaken.
Patients should be followed-up with routine colonoscopy and/or sigmoidoscopy, depending on the site of the primary tumor and according to local practice.
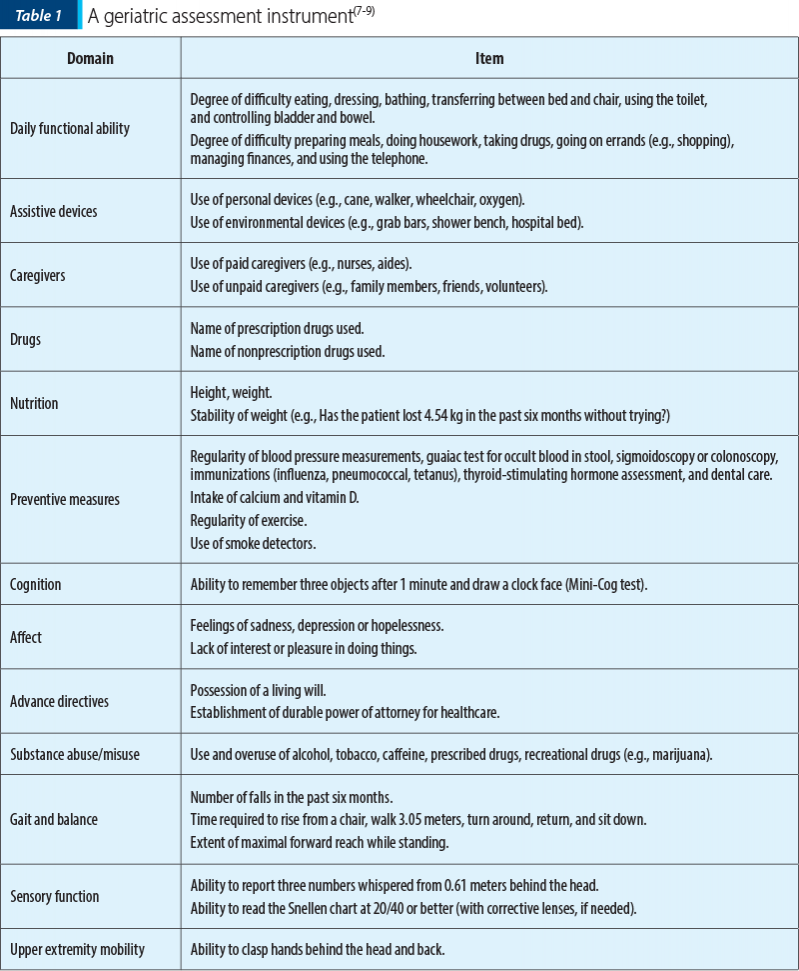
In terms of clinical assessment, SIOG recommended that, due to the heterogeneity of the elderly patient population, adults with colorectal cancer above 65 years of age needed to undergo a preoperative evaluation. This evaluation should include a cancer-specific assessment, as well as a whole patient evaluation for the most common physiological side-effects of aging, physical and mental ability and social support. In the case of a patient with physical or psychological comorbidities measured by the comprehensive geriatric assessment (CGA) or by local screening methodology, a geriatrician should be involved in patient management.
Comprehensive geriatric assessment is a multidimensional process designed to assess the functional ability, health (physical, cognitive and mental), and socioenvironmental situation of older people.
Precision cancer medicines
RAS oncogene was studied in colon cancer, because that gene is implicated in the development and evolution of this type of cancer. Mutations in the RAS genes result in permanently “turned on” switches which in turn result in uninhibited cell division, that can lead to cancer. There are three types of RAS oncogenes, designated NRAS, GRAS, and KRAS. KRAS mutations are the most common oncogenic alteration in all of human cancers, and there are currently no effective treatments available for patients with KRAS-mutant cancers.
The majority of patients with pancreatic or colon adenocarcinoma carry a mutation or have the amplification of KRAS. Several drugs are in development to target RAS and KRAS mutations, including KRASG12C and KRASG12D.
Onvansertib. The U.S. Food and Drug Administration (FDA) granted Fast Track Designation to onvansertib, an orally administered highly selective polo-like kinase 1 (PLK1) inhibitor that is being developed in patients with KRAS-mutated metastatic colorectal cancer.
Berzosertibe is a first-in-class ATR inhibitor M6620 (VX-970) that blocks a key DNA repair protein called ATR which is undergoing clinical evaluation as a single agent or in combination with chemotherapy in an early-phase clinical trial(9).
Adagrasib. The KRAS G12C inhibitor drug Krazati® (adagrasib, MRTX849) joins Lumakras® (sotorasib) as an approved treatment for patients with non-small cell lung cancers (NSCLC) harboring KRAS G12C mutations. These drugs are also being evaluated in colorectal cancer and other solid tumors(10-11).
MRTX113 is a KRASG12D inhibitor in development by Mirati.
ELI-002 is a structurally novel investigational vaccine targeting KRAS-driven cancers. ELI-002 is comprised of AMP-modified mutant KRAS peptide antigens and ELI-004, an AMP-modified immune-stimulatory oligonucleotide adjuvant. The AMP mKRAS peptides and AMP CpG are targeted to the lymph node where they potentially enhance action on key immune cells. ELI-002 targets the seven most common KRAS mutations and has the potential to become a multi-targeted mKRAS therapy that can prevent disease recurrence in patients with KRAS-driven tumors.
VS-6766 blocks tumor growth signaling downstream of mutant KRAS potentially, creating a more complete and durable antitumor response through maximal RAS pathway inhibition. VS-6766 is being evaluated alone and with the FAK inhibitor defactinib which blocks KRAS signaling. FAK has been identified preclinically and clinically as a potential resistance mechanism to RAF and MEK inhibition.
SHP2 inhibitors. Although some KRAS inhibitors have been demonstrated to be effective, most cancer cells ultimately develop adaptive resistance to medicines targeting KRAS. SHP2 is an oncogenic tyrosine phosphatase involved in signal transduction. Effective targeting of SHP2 has the potential to overcome resistance in RAS/MAPK-driven cancers. ETS001 and RMC-4630 are entering clinical trials and may effectively treat RAS/MAPK-altered tumors either alone or in combination with other targeted therapies(12).
Drugs for wild-type KRAS
Wild-type KRAS represent about 10% of all cases, maybe even more among younger patients.
Nimotuzumab is an antibody that targets epidermal growth factor receptor (EGFR) and blocks the EGFR pathway, inhibiting tumor growth. It is currently available in China and Germany, but not in the USA. The results of 92 patients with wild-type KRAS advanced pancreatic cancer treated with nimotuzumab + Gemzar® (gemcitabine) compared to Gemzar® alone were reported at ASCO 2022. Nimotuzumab-Gemzar® treated patients had a longer average overall survival duration of 10.9 months compared with 8.5 months for Gemzar® alone, and 43.6% of nimotuzumab treated patients survived one year compared with 2.7% with Gemzar®(13).
Drugs for BRAF mutated gene
BRAF is mutated in approximately 3% of patients with pancreatic cancer, and can be treated with BRAF/MEK inhibitor combinations. The BRAF and MEK genes are known to play a role in cell growth, and mutations of these genes are common in several types of cancer. A combination of a BRAF and a MEK inhibitor, tested first in melanoma, appears to decrease the emergence of disease resistance that occurs in patients with a BRAF mutation(14).
Conclusions
The systemic treatment of advanced colon cancer is complex. Because this cancer affects especially older patients, it is important to take into consideration the geriatric consult and assessment. Another conclusion is that oxaliplatin for some elder patients is not efficient and gives only toxicity. Gene determination is very important to establish the prognostic and treatment of patients with colon cancer. For example, the treatment with KRAS inhibitors (panitumumab) is better indicated by negative hyperselection using ctDNA rather than tumor sidedness.
Corresponding author: Alexandru Grigorescu E-mail: alexgrigorescu2004@yahoo.com
Conflict of interest: none declared.
Financial support: none declared.
This work is permanently accessible online free of charge and published under the CC-BY licence.
Bibliografie
-
Innocenti F, Mu W, Qu X, et al. DNA Mutational Profiling in Patients with Colorectal Cancer Treated with Standard of Care Reveals Differences in Outcome and Racial Distribution of Mutations. J Clin Oncol. 2024;42(4):399-409.
-
Findings from an exploratory preplanned biomarker analysis of the PARADIGM study. 16 February 2024. https://www.esmo.org/oncology-news/a-lack-of-resistance-gene-alterations-in-baseline-ctdna-associated-with-prolonged-os-after-first-line-treatment-with-panitumumab-plus-chemotherapy-in-patients-with-ras-wt-mcrc?utm_source=SciNews&utm_medium=email&utm_campaign=ESMO-WW-MED-Members-Scientific-enews-email-SciNews-20240222
-
Shitara K, Muro K, Jun Watanabe J, et al. Negative hyperselection of patients with RAS wild-type metastatic colorectal cancer for panitumumab: A biomarker study of the phase III PARADIGM trial. Journal of Clinical Oncology. 2023;41(Suppl_4).
-
Oxaliplatin in Older Adults With Resected Colorectal Cancer?. https://www.medscape.com/viewarticle/999630?form=fpf
-
Quasar Collaborative Group, Gray R, Barnwell J, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007;370(9604):2020-2029 .
-
COLOXIS: A Machine Learning Model that Predicts Oxaliplatin Benefit in Colon Cancer Treatment. https://medriva.com/cancer/coloxis-a-machine-learning-model-that-predicts-oxaliplatin-benefit-in-colon-cancer-treatment/
-
Papamichael D, Audisio R, Horiot JC, et al. Treatment of the elderly colorectal cancer patient: SIOG expert recommendations. Ann Oncol. 2009;20(1):5-16.
-
MSD Manual. Stefanacci RG. Comprehensive Geriatric Assessment Manual, professional version. Reviewed/Revised May 2022. Modified Sep 2022. https://www.msdmanuals.com/professional/geriatrics/approach-to-the-geriatric-patient/comprehensive-geriatric-assessment
-
https://news.cancerconnect.com/pancreatic-cancer/drugs-in-development-for-advanced-pancreatic-colon-cancers
-
Spira IA, Riely GJ, Gadgeel SM, et al. KRYSTAL-1: Activity and Safety of Adagrasib (MRTX849) in Advanced/Metastatic Non-Small-Cell Lung Cancer (NSCLC) Harboring KRASG12C Mutation. Journal of Clinical Oncology. 2022 June;40(Suppl_16).
-
Johnson M, Ou SHI, Barve M, et al. KRYSTAL-1: Activity and Safety of Adagrasib (MRTX849) in Patients with Colorectal Cancer (CRC) and Other Solid Tumors Harboring a KRAS G12C Mutation. New Approaches to an Old Problem Scientific Session. European Journal of Cancer. 2020 Oct;138(Suppl_2):S2.
-
Wang Y, Mohseni M, Grauel A, et al. SHP2 blockade enhances anti-tumor immunity via tumor cell intrinsic and extrinsic mechanisms. Sci Rep. 2021;11(1):1399.
-
Ramakrishnan MS, Eswaraiah A, Crombet T, et al. Nimotuzumab, a promising therapeutic monoclonal for treatment of tumors of epithelial origin. MAbs. 2009;1(1):41-48.
-
Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371(20):1877-1888.






