New changes in the classification of acute myeloid leukemia proposed by WHO 2022
Actualităţi în clasificarea OMS 2022 privind încadrarea leucemiilor acute mieloide
Abstract
The new revision of WHO (World Health Organization) 2022 classification of acute myeloid leukemia (AML) aims to highlight the new data obtained mainly in the recent years regarding the pathogenesis of this disease. It stands out the removal of the 20% blast cutoff for the diagnosis of AML subtypes with defined genetic abnormalities, except for AML with BCR::ABL1 fusion and AML with CEBPA mutation. Also, we mention the elimination of the AML, NOS (not otherwise specified) category, a slightly confusing terminology because this subtype was characterized according to morphological differentiation, therefore the introduction of the new category called AML defined by differentiation seems more suitable. Another category, called AML with other defined genetic alterations, is introduced, an entity that will include new subtypes of AML that have the potential to become defined subtypes in the future. Regarding AML, myelodysplasia-related (AML-MR), it excludes morphology as the single diagnostic criterion, revising the defining cytogenetic abnormalities, and introducing eight defining mutations for the diagnosis of AML-MR. Also, it is observed the reclassification of the category of therapy-related myeloid neoplasms into the entity of secondary myeloid neoplasms, along with the category of myeloid proliferations associated with Down syndrome, a separate entity defined earlier by the 2016 WHO classification.Keywords
acute myeloid leukemia (AML)AML with defined genetic abnormalitiesAML defined by differentiationAML myelodysplasia-related (AML-MR)World Health Organization 2022 (WHO 2022)AML with other defined genetic alterationssecondary myeloid neoplasmsmyeloid neoplasms post-cytotoxic therapies (MN-pCT)Rezumat
Noua revizuire a clasificării OMS (Organizaţia Mondială a Sănătăţii) din 2022 a leucemiilor acute mieloide (LAM) are ca scop evidenţierea noilor date obţinute în ultimii ani cu privire la patogeneza bolii. Se remarcă eliminarea cutoff-ului de 20% blaşti pentru diagnosticul subtipurilor de LAM cu anomalii genetice definite, cu excepţia LAM cu fuziune BCR::ABL1 şi LAM cu mutaţia CEBPA. De asemenea, se observă eliminarea categoriei LAM, NOS (not otherwise specified), terminologie uşor derutantă, deoarece subtipurile erau caracterizate în funcţie de diferenţierea morfologică, şi introducerea categoriei denumite LAM definită prin diferenţiere. Se introduce o nouă categorie, numită LAM cu alte alterări genetice definite, categorie care va cuprinde noile subtipuri de LAM care au potenţial să devină subtipuri definite în viitor. În ceea ce priveşte LAM asociată mielodisplaziei (LAM-MR), se exclude morfologia drept criteriu unic de diagnostic, se revizuiesc anomaliile citogenetice definitorii şi se introduce un set de opt mutaţii somatice definitorii pentru diagnosticul de LAM-MR. Se observă translarea categoriei de neoplasme mieloide asociate terapiei în categoria de neoplasme mieloide secundare, la care se adaugă şi categoria de proliferări mieloide asociate cu sindromul Down, entitate separată în clasificarea OMS 2016.Cuvinte Cheie
leucemie acută mieloidă (LAM)LAM cu anomalii genetice definiteLAM definită prin diferenţiereLAM asociată mielodisplaziei (LAM-MR)Organizaţia Mondială a Sănătăţii 2022 (OMS 2022)LAM cu alte alterări genetice definiteneoplasme mieloide secundareneoplasm mieloid post-terapii citotoxice (MN-pCT)Introduction
The 2022 World Health Organization (WHO) classification of acute myeloid leukemia (AML) continues to integrate the morphology, immunophenotyping, cytogenetics and molecular biology to establish the positive diagnosis. However, the emphasis on defining it in more details through genetic changes involved in AML pathology is noteworthy, this being a step that will allow the development of personalized therapy in the future.
This new classification of acute myeloid leukemias includes the following categories: acute myeloid leukemia with defined genetic abnormalities, acute myeloid leukemia defined by differentiation, and myeloid sarcoma (Table 1), a classification to which secondary myeloid neoplasms are added(1).
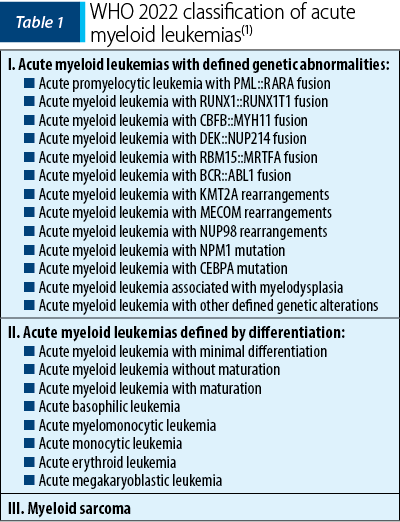
Acute myeloid leukemia with defined genetic abnormalities
The most important change that WHO 2022 makes in this category of AML is the elimination of the blast cutoff for establishing the diagnosis.
In WHO 2016, the diagnosis of AML was determined by the presence of ≥20% blasts in the bone marrow or peripheral blood, the only subtypes that did not require this criterion being acute promyelocytic leukemia (APL) with the PML::RARA fusion and AML with the fusion RUNX1-RUNX1T1 or CBFB-MYH11(2).
At this time, the cutoff of 20% blasts in the bone marrow and/or peripheral blood is the removed for all disease subtypes except for AML with BCR::ABL1 fusion and AML with CEBPA mutation, entities that still require ≥20% blasts to establish the positive diagnosis. The cutoff ≥20% blasts for AML with the BCR::ABL1 fusion is maintained because of the difficulty to differentiate it from the blast phase of chronic myeloid leukemia; in the case of AML with CEBPA mutation, there is not yet enough information to support the removal of this cutoff(1).
Regarding APL with the PML::RARA fusion and AML with the RUNX1-RUNX1T1 or CBFB-MYH11 fusion, the diagnostic criteria have not been changed, but the importance of the improvement in the evaluation techniques of minimal residual disease (MRD) is emphasized, as well as the importance of additional molecular abnormalities that becomes necessary for the patient management and appropriate treatment selection. Likewise, even in the case of AML with the DEK::NUP214 or RBM15::MRTFA fusion, the diagnostic criteria do not change(1).
Within the three subtypes of AML with gene rearrangements involving KMT2A, MECOM and NUP98, a blast count below 20% is accepted for the diagnosis, because multiple studies have shown that the clinical manifestations are similar to those of the patients with high blast counts. The identification of the fusion partner is not necessary, but its identification could provide additional information regarding the prognosis and management of the disease(1).
AML with NPM1 and CEBPA mutation also do not require a blast count criterion for the diagnosis. Multiple studies showed that MDN or MDN/MPN with NPM1 transform into AML very quickly, information which sustains the removal of the blast cutoff for diagnosis. In the case of AML with CEBPA mutation, biallelic mutations (biCEBPA) are accepted, as well as a single mutation located at the level of the basic leucine zipper (bZIP) region of the gene (smbZIP-CEBPA); the latter is associated with a favorable prognosis(1,3).
Another change in the new classification is the elimination of the provisional entity AML with RUNX1 mutation, due to the lack of data to support its strength as a single entity(4).
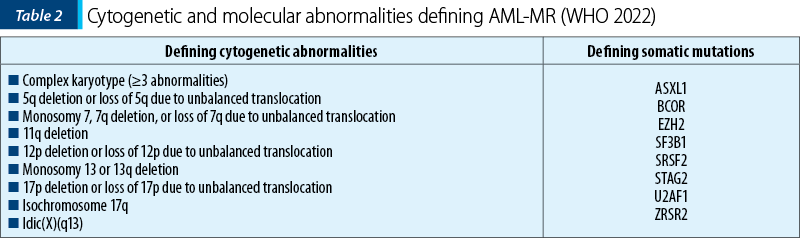
In the context of acute myeloid leukemia, myelodysplasia-related (AML-MR), previously named AML with myelodysplastic changes, WHO introduces multiple changes. The diagnosis requires the presence of ≥20% blasts expressing myeloid immunophenotyping and the presence of one or more specific cytogenetic or molecular abnormalities associated with myelodysplastic neoplasms (MDN), defining AML which is de novo or post-MDN or post-MDN/MPN (myelodysplastic neoplasm/myeloproliferative neoplasm). Thus, the morphology is excluded as the unique criterion for diagnosis, the defining cytogenetic abnormalities are revised, and eight defining mutations are introduced for the diagnosis of AML-MR(1). The defining cytogenetic abnormalities for this category of AML remain the same as in the 2016 WHO classification, except for the balanced cytogenetic abnormalities which have been subsequently removed. The defining somatic mutations for AML-MR are as follows: ASXL1, BCOR, EZH2, SF3B1, SRSF2, STAG2, U2AF1 and ZRSR2. These mutations show >95% specificity for AML post-MDN or post-MDN/MPN(1,2).
AML with other defined genetic alterations is a new category that is characterized by the inclusion of new subtypes of AML or rare AML subtypes that may become defined subtypes in the future. Currently, the following AML with other defined genetic alterations have been described: AML with RUNX1T3(CBFA2T3)::GLIS2, AML with KAT6A::CREBBP, AML with FUS::ERG, AML with MNX1::ETV6, AML with NPM1::MLF(1,5).
Acute myeloid leukemia defined by differentiation
AML defined by differentiation, previously known as AML, NOS (not otherwise specified), is removed due to slightly confusing terminology, considering that AML subtypes were characterized according to morphological differentiation. This category is characterized by cases of AML that do not present defined genetic abnormalities. It is estimated that, with the progress of research in the field of genetic studies, the cases within this category of AML will gradually decrease(1).
The subtypes of AML defined by differentiation are as follows: AML with minimal differentiation, AML without maturation, AML with maturation, acute basophilic leukemia, acute myelomonocytic leukemia, acute monocytic leukemia, acute erythroid leukemia, and acute megakaryoblastic leukemia.
This category still requires ≥20% blasts in bone marrow and/or blood (except for acute erythroid leukemia that requires ≥30% proerythroblasts). It does not meet the criteria for any AML type with defined genetic alterations or the criteria for mixed-phenotype acute leukemia, and it does not fulfill the diagnostic criteria for myeloid neoplasm post-cytotoxic therapy, or any prior history of myeloproliferative neoplasm.
Acute erythroid leukemia is characterized by ≥80% of bone marrow infiltration with erythroid predominance, of which ≥30% are proerythroblasts (or pronormoblasts) and a high presence of biallelic TP53 alterations. At this moment, the mutations within TP53 are underestimated. Also, in acute megakaryoblastic leukemia, there have been detected newly described gene fusions, namely CBFA2T3::GLIS2, a subtype of AML with other defined genetic alterations.
Myeloid sarcoma
Myeloid sarcoma is an isolated extramedullary tumor arising as a manifestation of AML or in the case of transformation of MDN, MDN/MPN or MPN. It is recommended that de novo myeloid sarcoma be further investigated, including both cytogenetic and molecular examination, for a better classification and an appropriate therapeutic plan. Molecular changes in myeloid sarcoma occurring concurrently with bone marrow involvement are concordant in 70% of cases, which is why myeloid sarcoma is considered to derive from a common hematopoietic stem cell or its precursor(1).
Secondary myeloid neoplasms
This category defines diseases that appear in the context of known precipitating factors, being represented by myeloid neoplasms secondary to exposure to cytotoxic therapies (MN-pCT) such as chemotherapy or radiotherapy, and myeloid neoplasms associated with germline predisposition, this last subcategory also including myeloid proliferations associated with Down syndrome, a separate entity in the 2016 WHO classification. Thus, acute myeloid leukemia secondary to cytotoxic therapy and AML associated with germline predisposition are now part of this category. Also, the category of MN-pCT includes myeloid neoplasms arising in the context of the use of PRAP1 inhibitors. Likewise, AML secondary to transformation from MPN remains in the category of MPN – blast phase, and those transformed from MDN or MDN/MPN remain in the category of AML-MR(1).
It is recommended to add the annotation “post-cytotoxic therapy” or “associated with the germline variant (gene)” to the writing of the diagnosis, for example: APL with the fusion of PML::RARA post-cytotoxic therapy; AML with germline variants in the RUNX1 mutation(1).
Conclusions
The changes that have been recently introduced in the classification of AML emphasize a step forward to a better understanding of the pathogenesis, especially through the genetic studies. This can only stand as an improvement to our daily practice, that stimulates the future of personalized therapies.
Conflict of interest: none declared.
Financial support: none declared.
This work is permanently accessible online free of charge and published under the CC-BY licence.
Bibliografie
-
Khoury JD, Solary E, Abla O, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia. 2022;36(7):1703-1719.
-
Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405.
-
The College of American Pathologists. What’s new in AML Classification (WHO 2022 vs International Consensus Classification). Accessed November 27, 2023. https://www.cap.org/member-resources/articles/whats-new-in-aml-classification-who-2022-vs-international-consensus-classification
-
Classification of acute myeloid leukemia (AML). UpToDate. Accessed November 27, 2023. https://www.uptodate.com/contents/classification-of-acute-myeloid-leukemia-aml
-
The New WHO Classification 2022. Accessed November 28, 2023. https://www.mll.com/en/the-new-who-classification-2022





