This short review refers to the main studies presented at the ASCO and ESMO 2023 congresses on digestive cancers. From the analysis of these studies, it appears that chemotherapy, especially in pancreatic cancer, remains useful, and the ideal chemotherapy formula has not yet been precisely established, most likely having to be adapted to each individual patient. Immunotherapy finds its place in the therapeutic strategy of cancers of the digestive sphere, with advanced studies in liver cancer and colon cancer. Targeted therapy also has its established place in therapeutic schemes, depending on biomarkers. A new concept on cancer therapy in general that also applies to digestive cancers is the theory of “cellular senescence”, influenced by genetic and immunological factors, a theory that could solve the cellular heterogeneity of malignant tumors that sometimes makes systemic therapy ineffective.
Some important trials regarding the digestive cancers presented at ASCO and ESMO congresses with impact in current medical practice
Trialuri importante privind cancerele digestive, prezentate la congresele ASCO şi ESMO, cu impact în practica medicală curentă
First published: 18 decembrie 2023
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/OnHe.65.4.2023.8956
Abstract
Rezumat
Acest scurt review se referă la principalele studii prezentate la congresele ASCO şi ESMO din 2023, având ca subiect cancerele digestive. Din analiza acestor studii rezultă că chimioterapia, în special în cancerul pancreasului, rămâne utilă şi încă nu s-a stabilit cu exactitate formula ideală de chimioterapie, cel mai probabil aceasta trebuind adaptată fiecărui pacient în parte. Imunoterapia îşi găseşte locul în strategia terapeutică a cancerelor sferei digestive, cu studii avansate în cancerul hepatic şi cancerul de colon. Terapia ţintită îşi are, de asemenea, locul stabilit în schemele terapeutice, în funcţie de biomarkeri. O nouă concepţie asupra terapiei cancerelor în general, care se aplică şi în cancerele digestive, este teoria „senescenţei celulare”, influenţată de factori genetici şi imunologici, teorie care ar putea rezolva eterogenitatea celulară a tumorilor maligne, care uneori face ineficientă terapia sistemică.
ASCO Congress
Addition of anti-TIGIT agent in unresectable liver cancer
“The strategies used for immune checkpoint inhibitor targeting cancer therapies have been influenced by some significant successes, including blocking the advanced-stage malignant tumor by death protein 1 (PD-1)/programmed cell death ligand (PDL-1), and cytotoxic T-lymphocyte antigen-4 (CTLA4) pathways. T cell immunoreceptor with immunoglobulin and ITIM domains (TIGIT) is an immune checkpoint that participates in tumor immune surveillance. Mainly expressed on T cells, natural killer (NK) cells and other antigen-presenting cells (APCs), it diminishes cytokine production and exhibits strong suppressive properties. TIGIT achieves a more active antitumor immune response and highlights a pivotal role for cancer immunotherapy. Preclinical studies have found inhibitory effects using a targeted approach.
Monotherapy targeting TIGIT or in combination with anti-PD-1/PD-L1 monoclonal antibodies for the treatment of patients with advanced solid malignancies has demonstrated improved antitumor immune responses. Due to the high tumor heterogeneity of liver cancer, immune checkpoint suppression therapy still needs further exploration.”(1)
A new immune target in cancer is TIGIT (also called WUCAM, Vstm3, or VSIG9) which is a receptor of the Ig superfamily, that plays a critical role in limiting adaptive and innate immunity.
TIGIT, this new immune target in cancer, participates in a complex regulatory network competing costimulatory receptor (DNAM-1/CD226) and multiple ligands – e.g., CD155 (PVR/NECL-5), CD112 (Nectin-2/ PVRL2(2-5).
In the phase Ib/II MORPHEUS-Liver study, in hepatocellular carcinoma, the addition of the novel agent tiragolumab to standard-of-care atezolizumab plus bevacizumab improved the responses as a first-line treatment. The findings point to anti-TIGIT treatment as a potential enhancer of checkpoint inhibitors, according to Richard S. Finn, from the University of California, Los Angeles, and the Jonsson Comprehensive Cancer Center: “The triplet of tiragolumab, atezolizumab and bevacizumab boosted response rates, which were 11.1% with atezolizumab plus bevacizumab and 42.5% with the addition of the anti-TIGIT compound. At a median follow-up of 14.3 months for the triplet and 12.1 months for standard therapy, investigator-assessed median progression-free survival was 11.1 months versus 4.2 months (hazard ratio [HR] = 0.42), respectively. Grade 3 or 4 adverse events were recorded in 50.0% of the control arm and 40.0% of the triplet arm; 16.7% and 15%, respectively, were grade 5, and those leading to withdrawal occurred in 5.6% and 12.5%, respectively. All-grade immune-mediated rash increased in frequency with the addition of tiragolumab (47.5% versus 33.3%), but there were no instances of grade 3 or 4 immune-mediated rash in either arm.”(1)
Neoadjuvant therapy for colon cancer
In this respect, a study presented by a Danish team is the randomized phase III NeoCol trial. Neoadjuvant chemotherapy, in this trial, was not superior to standard upfront surgery and adjuvant therapy in patients with colon cancer, but it did offer some relative benefits, according to Lars Henrik Jensen, from the Danish Colorectal Cancer Center South, University Hospital of Southern Denmark, Vejle.
Some advantages of neoadjuvant surgery were presented by Dr. Jensen: “Surgery proved to be a safe procedure after neoadjuvant chemotherapy. The absolute numbers are small, but the relative risk of ileus or anastomotic leakage favored neoadjuvant chemotherapy”(6).
“For upfront surgery versus neoadjuvant surgery, respectively, ileus was seen in 8% and 3%, and anastomotic leakage, in 8% and 2%. Tumors were downstaged with neoadjuvant treatment, with 3% of patients achieving complete responses and 7% achieving in situ status or better; no patient in this arm had lymph node spread, and few patients had vascular invasion. Significantly fewer patients in this arm needed adjuvant therapy”(6).
Some toxicity was more frequent in neoadjuvant therapy: “sensory neuropathy grade 3 or 4 was more common with upfront surgery (11% versus 7%) during treatment and during follow-up (5% versus 2%)”(6).
Presented: T-DXd in HER2-positive colorectal cancer
“According to the primary analysis of the multicenter phase II DESTINY-CRC02 trial, fam-trastuzumab deruxtecan-nxki (T-DXd) showed antitumor activity in patients with HER2-positive metastatic colorectal cancer at both 5.4 mg/kg and 6.4 mg/kg. Antitumor efficacy was observed irrespective of RAS mutation status or previous treatment with an anti-HER2 agent. The study assessed the efficacy and safety of these two doses of T-DXd in 82 patients with HER2-positive unresectable, recurrent or metastatic colorectal cancer. Patients’ tumors were RAS wild-type or mutated and BRAF wild-type, and patients had received a median of three to four prior lines of therapy, including prior anti-HER2 therapies if indicated. The primary endpoint was confirmed objective response rate by blinded independent central review”(13). The best response was seen in 37.8% of patients receiving 5.4 mg/kg. The safety profile was good: “All-grade interstitial lung disease and pneumonitis rates were lower at 5.4 mg/kg (8.4% vs. 12.8% with 6.4 mg/kg)”(13).
Neoadjuvant chemotherapy for rectal cancer
The seven-year results of the phase III PRODIGE 23 trial have shown that overall survival in locally advanced rectal cancer can be improved with neoadjuvant chemotherapy using modified FOLFIRINOX (leucovorin, fluorouracil, irinotecan, oxaliplatin) followed by chemoradiation, surgery and adjuvant chemotherapy, as compared with standard chemoradiation, surgery and adjuvant chemotherapy.
“The study enrolled 461 patients with cT3 or cT4, M0 rectal adenocarcinomas less than 15 cm from the anal verge. They were randomly assigned to the standard of care therapy (radiotherapy plus capecitabine, followed by total mesorectal excision, and then by adjuvant chemotherapy with modified FOLFOX6 [fluorouracil, leucovorin, oxaliplatin] or capecitabine), or neoadjuvant therapy with modified FOLFIRINOX (followed by radiotherapy plus capecitabine, then by total mesorectal excision, and then by adjuvant modified FOLFOX6 or capecitabine).”(14)
“TNT with modified FOLFIRINOX should now be considered as one of the best options in patients with locally advanced rectal cancers (...) This strategy is now being investigated in rectal cancer for tailored management, according to response to induction modified FOLFIRINOX.”(14)
PRODIGE 23 is the first randomized trial to demonstrate an overall survival benefit since the Swedish Rectal Cancer Trial on preoperative radiotherapy in 1997(7).
Pancreatic cancer
A phase 1/2 study (Wainberg, et al. Eur J Cancer. 2021;151:14–24; NCT02551991) demonstrated promising anti-tumor activity in patients with mPDAC who received first-line liposomal irinotecan 50 mg/m2 + 5-FU 2400 mg/m2 + LV 400 mg/m2 + oxaliplatin 60 mg/m2 (NALIRIFOX). Herein, we present the results from NAPOLI-3 (NCT04083235), a randomized, open-label, phase 3 study investigating the efficacy and safety of NALIRIFOX compared with nab-paclitaxel + gemcitabine as first-line therapy in patients with mPDAC.
The main results of the study are presented in Table 1.
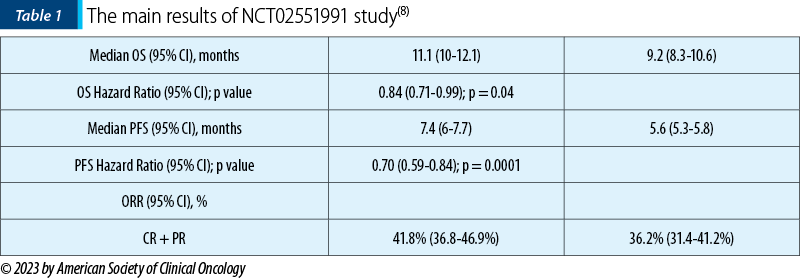
This study demonstrated a superiority in survival for NALIRIFOX versus nab-paclitaxel + gemcitabin(8).
Knut Jørgen Labori, MD, presented a phase II trial: “Neoadjuvant Approach Falls Short in Pancreatic Cancer in NORPACT-1”. The reviewer emphasizes the conclusions of the study authors: “In the multicenter randomized phase II NORPACT-1 trial of patients with resectable cancer of the pancreatic head, a short course of neoadjuvant FOLFIRINOX was not associated with improved overall survival compared with upfront surgery and adjuvant chemotherapy”(9).
The PROSPECT trial was a randomized phase III trial of neoadjuvant chemoradiation versus neoadjuvant FOLFOX chemotherapy with selective use of chemoradiation, followed by total mesorectal excision (TME) for the treatment of locally advanced rectal cancer (LARC) – Table 2.
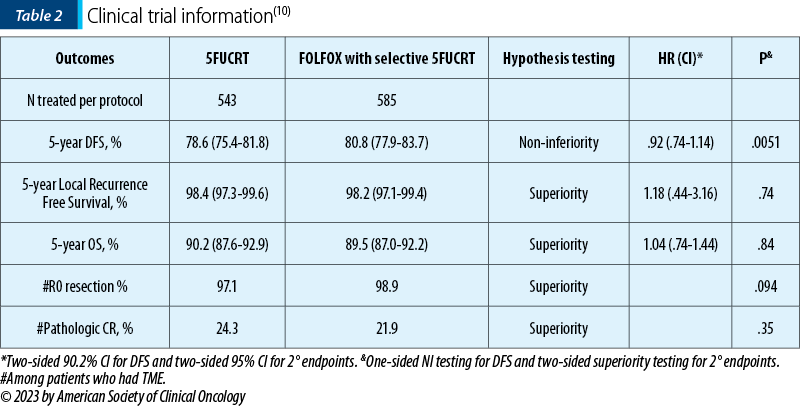
“Background. Radiation with sensitizing fluoropyrimidine (5FUCRT) is a standard curative intent treatment for LARC. It improves disease-free survival (DFS) by decreasing pelvic recurrence but has short- and long-term toxicity. The PROSPECT trial compares FOLFOX chemotherapy with selective use of 5FUCRT (intervention) to 5FUCRT (control) for neoadjuvant treatment prior to TME for LARC. (...) From June 2012 to December 2018, 1194 patients were randomized and 1128 initiated protocol-assigned treatment. Median age was 57; 34.5% were women and 61.9% had clinically positive nodes. 53 of 585 patients in the intervention group (9%) received preoperative 5FUCRT. DFS was analyzed after 227 events and median follow-up of 58 months. Conclusions. FOLFOX chemotherapy with selective use of 5FUCRT is non-inferior to 5FUCRT for neoadjuvant treatment of LARC prior to low anterior resection with TME. Patients and physicians have alternative strategies for the management of LARC.”(10)
ESMO Congress – important trials in gastrointestinal cancers
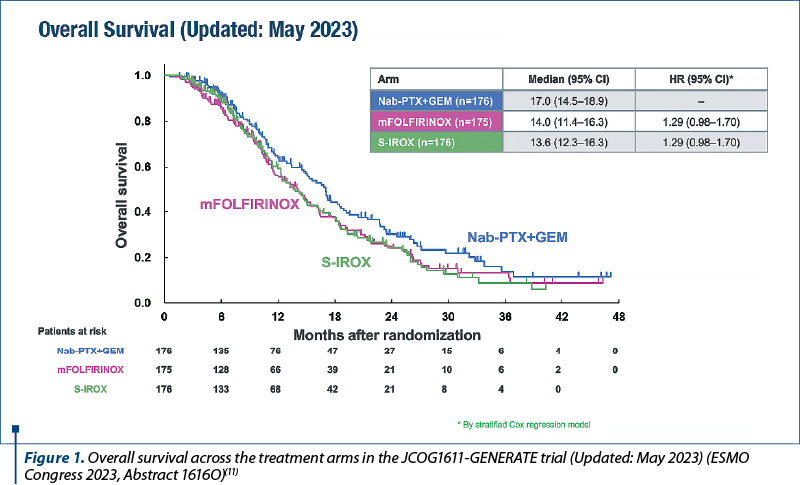
“The results from the JCOG1611-GENERATE trial fuel the debate around the preferred option in this setting.
As presented at the ESMO Congress 2023 (Madrid, 20-24 October), a planned interim analysis on 527 patients in the multicenter, randomized, open-label, phase II/III JCOG1611-GENERATE trial supports the use of nab-paclitaxel plus gemcitabine as first-line treatment for patients with metastatic or recurrent pancreatic cancer compared with modified FOLFIRINOX (oxaliplatin 85 mg/m2, irinotecan 150 mg/m2, l-leucovorin 200 mg/m2 and fluorouracil 2400 mg/m2 on days 1-3, every two weeks) or S-IROX (oxaliplatin 85 mg/m2, irinotecan 150 mg/m2 and S-1 80 mg/m2/day on days 1-7, every 2 weeks) (Abstract 1616O).
As Dr. Angela Lamarca highlights in her editorial, these findings open a very interesting debate about which therapy regimen may be preferable in this setting.
Investigators reported median overall survival times of 17 months with nab-paclitaxel (125 mg/m2) plus gemcitabine (1000 mg/m2), 14 months with modified FOLFIRINOX (hazard ratio [HR] 1.29; 95% confidence interval [CI]; 0.98-1.70) and 13.6 months with S-IROX (HR 1.29; 95% CI; 0.98-1.70) in May 2023. These updated data were similar to those from the planned analysis in March 2023 where the predictive probability for achieving superiority at the final analysis was calculated as 0.73% for the modified FOLFIRINOX arm and 0.48% for the S-IROX arm, leading to termination of the trial for futility. Both triplet therapies were also associated with a higher incidence of the most common grade ≥3 non-hematological toxicity (anorexia), with rates of 22.8% and 27.6% in the modified FOLFIRINOX and S-IROX arms, respectively, compared with 5.2% in the nab-paclitaxel plus gemcitabine arm.
The trial enrolled patients aged between 20 and 75 years old, with pathologically confirmed metastatic or recurrent pancreatic cancer and an Eastern Cooperative Oncology Group performance status of 0 or 1, who were randomized 1:1:1 to treatment at centers in Japan.”(11)
Conclusions
A new approach to treat gastrointestinal cancer is represented by the use of the tumor suppressive effect of cellular senescence, avoiding the tumor promoting effect, improving the sensitivity of treatment, and preventing tumor recurrence and metastasis.
Cell senescence is a permanent cell cycle arrest associated with a variety of secretory phenotypes. Induction of cell senescence is an important mechanism of tumor inhibition by chemoradiotherapy and targeted therapy.
The clinical efficacy of immunotherapy is, at least in part, caused by the killing of subpopulations of resistant cancer cells through a bystander effect. While the contribution of bystander effects in the context of senescence-inducing therapies is only poorly understood to date, it may represent an opportunity to overcome tumor heterogeneity(12).
The future studies will probably further explore the molecular mechanism of tumor cell senescence, further enrich the therapeutic targets of gastrointestinal cancer, and find new treatment strategies to improve the therapeutic effect.
Conflict of interest: none declared.
Financial support: none declared.
This work is permanently accessible online free of charge and published under the CC-BY licence.

Bibliografie
-
Zheng Q, Xu J, Gu X, et al. Immune checkpoint targeting TIGIT in hepatocellular carcinoma. Am J Transl Res. 2020;12(7):3212-3224.
-
Stanietsky N, Simic H, Arapovic J, et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc Natl Acad Sci USA. 2009;106:17858–63.
-
Yu X, Harden K, Gonzalez LC, et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. 2009;10(1):48–57.
-
Bottino C, Castriconi R, Pende D, et al. Identification of PVR (CD155) and nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J Exp Med. 2003;198(4):557–67.
-
Zhu Y, Paniccia A, Schulick AC, et al. Identification of CD112R as a novel checkpoint for human T cells. J Exp Med. 2016;213(2):167–76.
-
Jensen LA, Kjaer ML, Larse FO, et al. Phase III randomized clinical trial comparing the efficacy of neoadjuvant chemotherapy and standard treatment in patients with locally advanced colon cancer: The NeoCol trial. Journal of Clinical Oncology. 2023; 41:17_suppl, LBA3503.
-
Swedish Rectal Cancer Trial, Cedermark B, Dahlberg M, et al. Improved survival with preoperative radiotherapy in resectable rectal cancer [published correction appears in N Engl J Med 1997 May 22;336(21):1539]. N Engl J Med. 1997;336(14):980-987.
-
Wainberg ZA, Melisi D, Macarulla T, Pazo-Cid R, Chandana SR, De La Fouchardiere C. NAPOLI-3: A randomized, open-label phase 3 study of liposomal irinotecan + 5-fluorouracil/leucovorin + oxaliplatin (NALIRIFOX) versus nab-paclitaxel + gemcitabine in treatment-naïve patients with metastatic pancreatic ductal adenocarcinoma (mPDAC). Journal of Clinical Oncology. 2023 ASCO Gastrointestinal Cancers Symposium. 2023;41:4_suppl, LBA661.
-
Labori KJ, Bratlie SO, Biörserud C, et al. Short-course neoadjuvant FOLFIRINOX versus upfront surgery for resectable pancreatic head cancer: A multicenter randomized phase II trial (NORPACT-1). Journal of Clinical Oncology. 2023 ASCO Annual Meeting II. Abstract LBA4005. 2023;41:17_suppl, LBA4005.
-
Schrag D, Shi Q, Weiser MR, et al. PROSPECT: A randomized phase III trial of neoadjuvant chemoradiation versus neoadjuvant FOLFOX chemotherapy with selective use of chemoradiation, followed by total mesorectal excision (TME) for treatment of locally advanced rectal cancer (LARC) (Alliance N1048). Journal of Clinical Oncology. ASCO Annual Meeting II. 2023;41:17_suppl, LBA2.
-
Ohba A, Ozaka M, Ogawa G, et al. Pancreatic cancer, results from the JCOG1611-GENERATE trial (esmo.org). https://dailyreporter.esmo.org/esmo-congress-2023/gastrointestinal-cancers/triplet-therapy-does-not-outperform-doublet-treatment-first-line-in-metastatic-pancreatic-cancer
-
Wang L, Lankhorst L, Bernards R. Exploiting senescence for the treatment of cancer. Nat Rev Cancer. 2022;22(6):340-355.
-
Raghav KPS, Siena S, Takashima A, et al. Trastuzumab deruxtecan (T-DXd) in patients (pts) with HER2-overexpressing/amplified (HER2+) metastatic colorectal cancer (mCRC): Primary results from the multicenter, randomized, phase 2 DESTINY-CRC02 study. Journal of Clinical Oncology. 2023;41:16_suppl, 3501.
-
Conroy T, Etienne PL, Rio E, et al. Total neoadjuvant therapy with mFOLFIRINOX versus preoperative chemoradiation in patients with locally advanced rectal cancer: 7-year results of PRODIGE 23 phase III trial, a UNICANCER GI trial. Journal of Clinical Oncology. 2023;41:17_suppl, LBA3504.
Articole din ediţiile anterioare
Aromatase inhibitors arthralgia
Pain definition: “An unpleasant sensory and emotional experience associated with actual or potential tissue injury or described in terms of such da...
ESMO - Labelled Meeting
Research in Medical Oncology and Palliative Care
Ethics in clinical research
Acest scurt review prezintă câteva elemente ale istoriei trialurilor clinice şi ale introducerii reglementărilor de etică în cadrul bunei pr...
Tumorile neuroendocrine - diagnostic şi strategii terapeutice
NETs are rare diseases. There is a lack of robust source data on epidemiology Nothing exists to describe the overall European situation: Only i...