Stereotactic Radiation Therapy is a type of external beam radiation therapy that delivers high dose to a relatively small target volume in one or few fractions, a technique that requires extremely conformal dose distributions, so the highest dose being delivered centrally in the tumor volume with a rapid fall off of dose to spare the adjacent healthy tissue. The dose is prescribed to the tumor periphery, usually to the 80% isodose (60-90%), the aim being that 95% of the target volume (PTV) is conformally covered by the prescription isodose surface and 99% of the target volume (PTV) receives a minimum of 90% of the prescription. Stereotactic radiotherapy can be used as a primary therapy for early stage primitive cancer or metastatic tumors (cranial or extra-cranial tumors), for patients in a good health conditions, with minimum 6 months life expectancy, and relatively small tumors.
Radioterapia stereotactică - principii şi aspecte practice
Stereotactic Radiation Therapy - principles and practical aspects
First published: 07 martie 2017
Editorial Group: MEDICHUB MEDIA
Abstract
Rezumat
Radioterapia stereotactică este o formă de iradiere externă care distribuie într-o şedinţă unică sau în câteva şedinţe doze mari de iradiere în volume-ţintă mici, tratament ce necesită o distribuţie a dozei extrem de precisă, astfel încât doza maximă să fie distribuită în volumul tumoral şi să scadă rapid peritumoral pentru a proteja ţesuturile adiacente sănătoase. Doza este prescrisă la periferia tumorii, de regulă la izodoza de 80% (60-90%), cu scopul ca 95% din volumul-ţintă planificat (PTV) să fie acoperit de izodoza de referinţă şi 99% din acest volum să fie iradiat cu cel puţin 90% din doza prescrisă. Radioterapia stereotactică poate fi utilizată în tratamentul tumorilor primitive (stadii precoce) sau al metastazelor (craniale sau extracraniale) în cazul pacienţilor cu stare generală bună, cu speranţa de viaţă de minimum 6 luni şi cu tumori mici.
Stereotactic Radiation Therapy is a type of external beam radiation therapy that, contrary to conventional external beam radiotherapy (EBRT) which uses 5 small daily fractions per week (1.8-2 Gy per fraction) to irradiate large volumes, it delivers a high dose to a relatively small target volume in one or few fractions(1). Therefore, two aspects have to be discussed regarding Stereotactic Radiotherapy: a technical aspect, meaning the high treatment precision needed to irradiate small volumes, and a biological aspect, the effects of a very high-dose delivered in few fractions to the tumours and the organs at risk (radiobiology aspects).
Delineation of target volumes
The simulation, realised by Computed Tomography Scan (CT scan), must provide a clear patient radioanatomy images, needed for the OAR delineation and the visualisation of the tumour position and eventually motion (with breathing, peristaltic activity, or organ filling and emptying)(2). The patient has to be in a comfortable and reproductive position for the treatment. The scanned anatomical region should cover the target volume (tumour) and all organs at risk with at least 5-10 cm superior and inferior margins, but the margin must be more larger if non coplanar fields are intended to be used. The tomography slice thickness of 1-3 mm is usually recommended(3).
The International Commission on Radiation Units and Measurements (ICRU) defined in its Reports (50 and 62) three main volumes necessary to be considered in conformal radiotherapy planning(4,5).
1. Gross Tumour Volume (GTV) represents the palpable or visible/demonstrable extent of malignant tumour, and is identified by CT scan, eventually in combination with other radiologic images, like Magnetic Resonance Imaging (MRI) or Positron Emission Tomography (TEP-CT). The GTV is identical for classical external beam radiation therapy (EBRT) and stereotactic radiotherapy.
2. Clinical Target Volume (CTV) is an anatomical concept, and represents all tissue volume that contains macroscopic and subclinical microscopic malignant disease, which has to be eliminated to accomplish the curative objective. As stereotactic radiotherapy is indicated for small tumours (3 cm maximal diameter for brain tumour and up to 6 cm for extracranian tumour), usually no expansion is made around GTV to take account for microscopic disease extent. Without margins, the clinical target volume (CTV) becomes equal to gross tumour volume (GTV)(6,7,8).
3. Internal Target Volume (ITV) takes into account movements and variations in size and shape of the CTV relative to anatomic reference points; e.g., filling of bladder, movements of respiration. From this point of view, there are two different target volumes: intra cranial fixed tumours (brain metastasis) and extra cranial, especially thoracic and abdominal tumour, which show significant movement during and between the radiotherapy fractions. To reduce tumour movement during respiration (reduce internal margin) 3 methods are actually used: the breath holding technique, the restricted respiration techniques and respiratory gating technique. In the breath holding technique, the patient temporarily suspends its breathing (in the inspiration phase) following an audible or light signal, and the tumour is irradiated only in this respiratory phase(9). The restricted respiration technique uses abdominal compression (small abdominal pressing plate) to control the patient’s diaphragm movements and to reduce the tumour movement during respiration(10). The respiratory gating technique synchronizes irradiation with the respiratory phase (mainly the expiratory phase). The patients, instructed to perform shallow respiration with oxygen, breath freely but periodically. Sensors attached to the thoracic wall of the patient or gold markers introduced near the tumour monitor the real-time motion of the ITV(11).
4. Planning Target Volume (PTV) is a geometrical concept intended to consider the net effect of all the possible geometrical variations and inaccuracies, in order to ensure that the prescribed dose is actually absorbed in the CTV(5).
Beam parameters
Beam energy. Usually 6 MV photon beams are used, providing a reasonable compromise between beam penetration and penumbra, knowing that for small narrow beams the higher the beam energy, the larger the beam penumbra due to lateral electron transport in medium(12).
Beam shaping. To improve the conformity of target dose distribution and normal tissue sparing , 2.5 to 5 mm multileaf collimator (MLC) are used(13).
Beams arrangement. In principle, two major techniques exist:
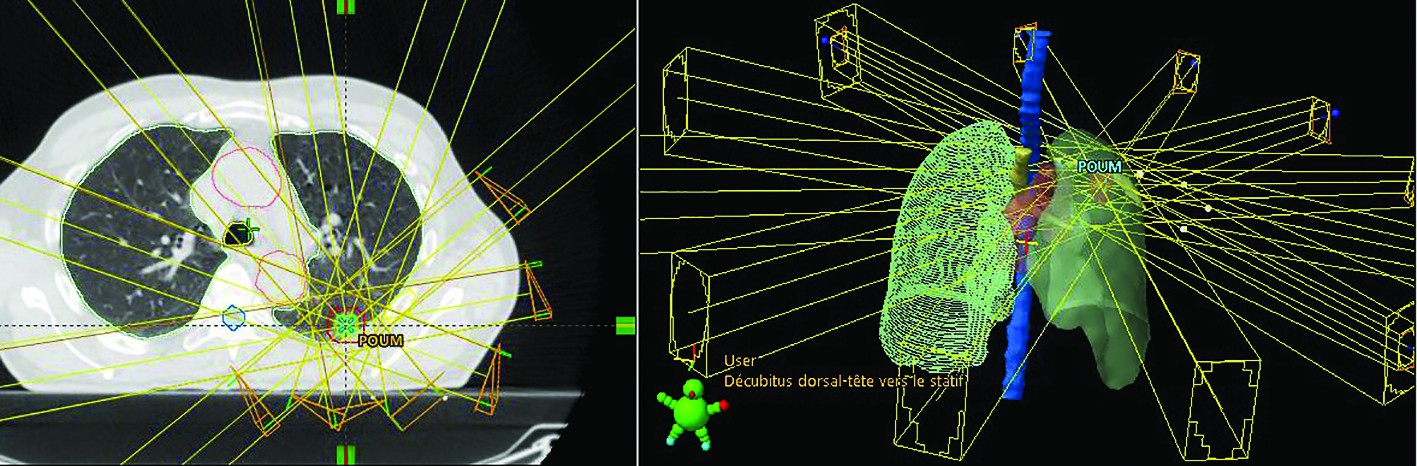
1. Fixed beams. Classical multiple static beams, coplanar or non-coplanar, whose number and arrangements have to be adjusted to confer the best dosimetric results. To avoid overlap dose between entrance and exit trajectories, these beams are ideally non opposing(14).
2. Rotational Beams. The basic concept of arc therapy is the delivery of radiation from a continuous rotation of the radiation source allowing the patient to be treated from a full 360° beam angle. There are two main forms of arc-based therapies: volumetric modulated arc therapy (VMAT) and helical IMRT in Tomotherapy(15). Volumetric modulated arc therapy (VMAT), possible on adapted conventional linear accelerators, delivers radiation with a single (up to 360 degree) or two gantry arcs. The gantry speed, multi-leaf collimator (MLC) position, aperture shape and fluence-output rate (dose rate) can dynamically vary during the rotation of the gantry(16). Tomotherapy, or Helical IMRT, is a form of computed tomography (CT) image-guided intensity-modulated radiotherapy that introduces the ring gantry concept into radiation oncology. The system, a combination of a therapeutic linear accelerator and a megavoltage CT-scanner, enables pre-treatment megavoltage CT imaging (before each radiotherapy fraction), which allows position control and correction, but also dose recalculations (doses distributions) for adaptative radiotherapy in case of geometry changes or tumour shrinkage(17).
Dosimetric parameters
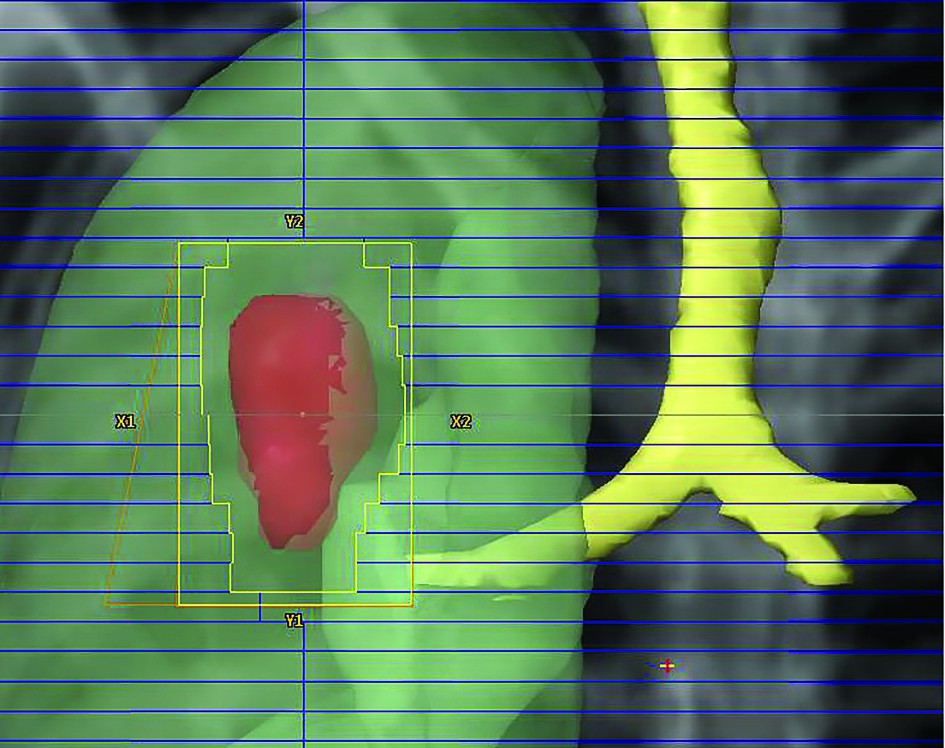
Dose prescription. For stereotactic radiotherapy, the dose can be prescribed to the isocenter as well as to the tumour (PTV) periphery, the last one being the clinically relevant dose because reflects the minimum dose received by tumour. Usually the dose is prescribed to the 80% isodose (ranging from 60-90%), the aim being that 95% of the target volume (PTV) is conformally covered by the prescription isodose surface and 99% of the target volume (PTV) receives a minimum of 90% of the prescription(18).
Dose heterogenity. Stereotactic radiotherapy requires extremely conformal dose distributions, the highest dose being delivered centrally in the target volume with a rapid fall off of dose in all directions near tumour periphery, to spare the healthy tissue(19).
Regarding the high dose and the low dose regions, the Radiation Therapy Oncology Group (RTOG) protocols recommend:
a) no hot spots (dose greater than 105% of the prescription) should be outside the PTV;
b) the fall off gradient beyond the PTV extending into normal tissue structures must be rapid in all directions(20).
Plan evaluation. Dosimetric indexes
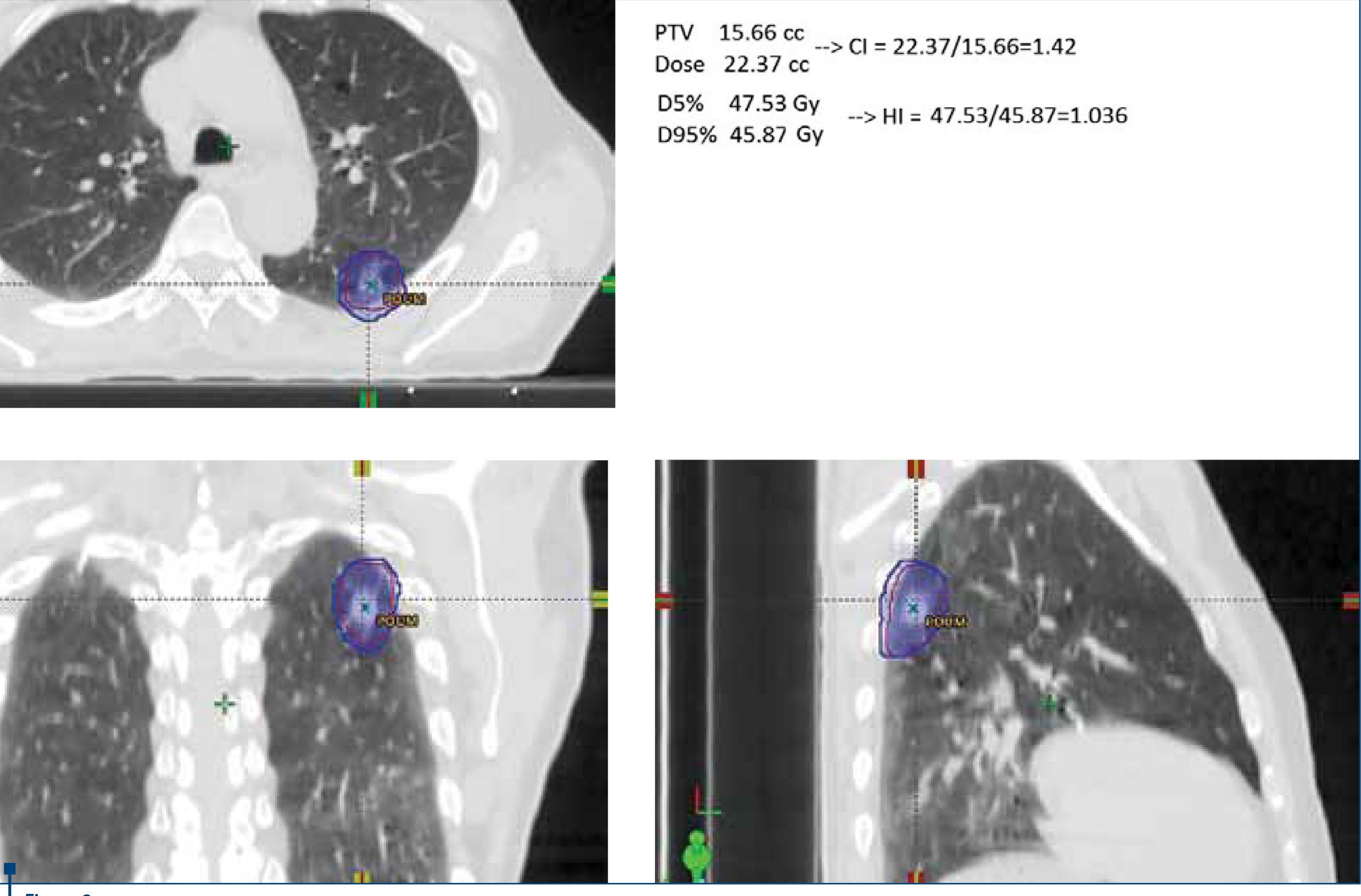
Conformity index (CI). It describes the quality of the target volume coverage, and is the ratio of two volumes, the prescription isodose volume (PIV) to the target volume (PTV). The ideal value for CI is 1(21).
Homogeneity index (HI). It is a measure of the uniformity of dose distribution in the target volume. The homogeneity index (HI) defined by RTOG protocols is the ratio of two doses, D5/D95 , where D5 = minimum dose in 5% of the Planning Target Volume (PTV), indicating the “maximum dose”, and D95 = minimum dose in 95% of the PTV, indicating the “minimum dose”. The dosimetric goal is to keep as low as reasonably achievable this ratio close to 1 if possible(22).
Dose gradient index (GI). Is a dosimetric parameter that can be used to compare treatment plans of equal conformity to show which of these prescription isodoses will give the steepest dose fall off outside of the target volume. The GI is defined as the ratio of two volumes, the volume of half the prescription isodose to the volume of the prescription isodose. For example, if the dose is prescribed to the 50% isodose line, the GI is the ratio of the 25% isodose volume to that of the 50% isodose volume(23).
Radiobiological aspects
The effectiveness of conventionally fractionated radiotherapy is directly influenced by 5 radiobiological determinants (5R): Reoxygenation (of hypoxic tumour cells), Repair (of sub-lethal radiation damages), Redistribution (of cells in cell cycle phases), Repopulation (of tumour cells) and Radiosensitivity (intrinsic sensitivity of tumour)(24).
Reoxygenation. Tumour oxygen concentration at the time of irradiation is an important radio-sensitivity factor, the hypoxia being a radio-resistance factor. Therefore, to obtain the same final effect (survival fraction) the hypoxic cells should be irradiated with a dose 2-3 times greater than necessary for oxygenated cells. This correction factor is named Oxygen Enhancement Radio(25). During fractionated conventional radiotherapy proportions of hypoxic cells are reoxygenated between radiation fractions, resulting in restoration of their radiosensitivity(26). A single fraction does not allow reoxygenation, but 6 to 8 radiation fraction can be reasonably efficient to benefit from reoxygenation phenomena(27).
Repair of sub-lethal radiation damage
Ionizing radiation is considered to produce two different types of lesion: non-repairable (i.e., lethal) lesions and repairable (i.e., potentially lethal) lesions. The reparation can be described by T1/2, the half time for repairing (time during which half of the DNA damage is repaired). The T ½ is usually about 30 minutes - 1 hour for cultured cells, but it may take 6-8 hours or longer in tissues (e.g., in CNS it may be more than 24 hours). The sublethal lesion repair is depending on the radiation dose, the interval between doses and the cellular repair capacity(28). When tumours are treated with stereotactic radiotherapy considerable repair of sublethal radiation damage may take place during the prolonged radiation exposure, for more than 30 minutes irradiation time the estimated loss of radiation effect is greater than 10%(29).
Redistribution (reassortment)
Cells exhibit differential radiation sensitivity dependent on the phases of the cell cycle, in mitosis being most sensitive and in late S-phase being most resistant(30). Consequently, surviving (radioresistant S-phase) cells progress into a more sensitive phase of the cell cycle, resulting as a therapeutic benefit(31). For moderate radiation doses the cell cycle arrest (predominantly in G2 phase ) is transitory, the distribution of cells through the cell cycle phases being restored to pre-irradiation state. After an exposure to high-doses of radiation, cells tend to be arrested indefinitely and die in the cell cycle phases in which they are irradiated (G1, S or G2 phase wherever they were at the time of irradiation)(29).
Repopulation
Repopulation of tumours, the proliferation of surviving clonogenic tumour cells during fractionated radiotherapy, is one of the most common causes of failure of conventional fractionated radiotherapy, because rapid multiplication of tumour cells between radiation fractions may in part compensate for the cell death(32).
For example, to compensate repopulation in rapid proliferative tumour (like head and neck, cervical, lung and oesophageal cancer), Royal College of Radiologists recommend a compensatory dose of 0.9 Gy per treatment day to be added after the 4th week of radiotherapy(33).
Radiosensitivity
The intrinsic radiosensitivity, which reflects the efficiency of DNA repair of Double-Strand Breaks (DSB) produced by irradiation, is a major factor responsible for the cancer outcome after conventional radiotherapy(34). The gold standard for evaluating ‘radiosensitivity’ has been the colony-survival assay (CSA), an in vitro radiation response evaluation, considered as a good surrogate for radiosensitivity(35). The parameter measuring the Surviving fraction at 2 Gy (SF2) has been correlated with the tumour radio-responsiveness both in mathematical and animal models. Concerning clinical studies, the SF2 was confirmed as an independent prognostic factor for radiotherapy outcome in cervix cancer (5-year survival of 81% for SF <0.42 versus 51% if SF >0.42) and head and neck cancers (5-year survival of 91% for SF <0.4 versus 74% if SF >0.4)(36,37).
The g-H2AX assay (Histone H2AX phosphorylation), a sensitive marker for DNA double-strand breaks (DSBs), is actually a novel radiosensitivity test. The g-H2AX assay is correlated with intrinsic radiosensitivity of human lymphocytes, fibroblasts and epithelial cells, a linear response proportional to the initial radiation dose being obtained 48 and 24 hours after exposure in blood samples and skin cells respectively(38).
For Stereotactic Radiotherapy, very few reports concerning intrinsic radiosensitivity are published. Three studies described the relationship between radiation necrosis risk after radiosurgery for arteriovenous malformations (AVM) and increased skin fibroblast radiosensitivity (clonogenic survival assays, SF2 0.10-0.20 for hypersensitive patients)(39,40,41). There is only one study regarding tumour radiosensitivity, a case report of an acoustic neuroma in a 78-year-old lady treated by stereotactic radiosurgery with 12 Gy tumour marginal dose (80% isodose). The clinical and radiological follow-up shows a dramatic reduction of the size of the tumour, from 27 mm initially to 20 mm at 19 months post-radiotherapy, suggesting a high intrinsic radiosensitivity. The 7-mm reduction at 19 month post-radiotherapy is considered significant because for acustic neuroma the typical response is stability in tumour size or minimal (1-2 mm), regression for 1-2 years followed by slow shrinkage (typically 1 mm/year)(42). This intrinsic radiosensitivity was confirmed by g-H2AX assay, which demonstrates defects in DNA repair mechanisms, incomplete DSB repair and therefore of increased radiosensitivity(42,43).
In addition to the tumour direct actions, Stereotactic Radiotherapy can produce indirect tumour cell death due to vascular damages and immune response. Vascular damage is responsible for secondary (indirect) cell death that occurs 2-3 days after high dose hypo-fractionated irradiation. This effect is dose-dependent, so the cell survival on days 2, 3, and 5 post-irradiation were similar to that immediately after irradiation for a 10 Gy irradiation, but markedly reduced for 15-20 or 30 Gy doses(44). Anti-tumour immunity is promoted/activated, 15 Gy or more increasing the trafficking of effector T-cells and the generation of CD9+ T-cells against the tumours and their distant metastases. The secondary (indirect) tumour cell death after high dose irradiation occurs within 1-3 days after irradiation whereas the full development of radiation-induced tumour-specific immunity usually takes for 1-2 weeks(45).
Treatment delivery
Stereotactic radiotherapy can be delivered by systems that require patient immobilization and/or a method to take account for any organ motion during treatment. In order to respect treatment accuracy in accordance with the treatment plan, on-board X-ray or CT images are acquired with the patient positioned on the treatment couch. These images are compared with the treatment plan images to ensure a match between the planning geometry and the treatment geometry. If the geometries do not match, the treatment table is readjusted(46). To monitor the motion of the tumour during treatment, a 1-2 mm gold (radio-opaque) marker is inserted into or near the periphery of tumours and then is detected by two sets of fluoroscopy. The treatment beam is gated to irradiate the tumour only when the position of the marker coincides with the planned tumour position(47). A non-invasive option is the use of external superficial markers. Instead of implantation of internal fiducial markers, the external surface motion is reconstructed on the trajectory of passive markers placed on the patient skin. The external surrogate method relies upon the assumption that the internal tumour motion is well correlated with the external respiratory induced motion, and that this correlation is constant in time(48). This method is not suitable for patients with medical or respiratory difficulty, while in fact, those patients actually need to be coached more than anyone else(49).
Stereotactic radiotherapy indications
Stereotactic radiotherapy can be used as a primary therapy for early stage cancer or as a targeted treatment for metastatic disease, for cranial or extra-cranial tumours (thorax, pancreas, liver, colon, uterus, pelvis, sacrum, kidney, prostate). Patient inclusion criteria for Stereotactic Radiotherapy include:
a) a minimum Karnofsky Performance Status (KPS) or World Health Organization (WHO), on average 70-100% KPS corresponding to 0-1 on WHO scale;
b) a minimum 6-month life expectancy;
c) well circumscribed tumour with a maximal diameter of 3-4 cm (for single fraction) and up to 6-7 cm (for multiples fractions);
d) a minimum distance between tumour and significant organs at risk (spinal cord, brain stem, optic chiasma and nerves etc.)(50).
In conclusion, Stereotactic Radiotherapy is a very precise irradiation technique, which uses single or multiples (usually 1-5) large dose fractions to treat relatively small cranial and extra cranial tumours. This treatment delivers precise and conformal high doses to the target volume and minimizes toxicity to normal tissues and critical organs at risk.
Bibliografie
2. E. Rietzel, T. Pan, G.T. Chen. Four-dimensional computed tomography: Image formation and clinical protocol. Med Phys, 32 (2005), pp. 874–889.
3. Prabhakar R, Ganesh T, Rath GK, Julka PK, Sridhar PS, Joshi RC, Thulkar S. Impact of different CT slice thickness on clinical target volume for 3D conformal radiation therapy. Med Dosim. 2009 Spring; 34(1):36-41.
4. ICRU. Prescribing, Recording and Reporting Photon Beam Therapy. Report 50. Bethesda, MD: International Commission on Radiation Units and Measurements, 1999. http://www.icru.org/home/reports/prescribing-recording-and-reporting-photon-beam-therapy-report-50
5. ICRU. Prescribing, Recording and Reporting Photon Beam Therapy (Supplement to ICRU Report 50). Report 62. Bethesda, MD: International Commission on Radiation Units and Measurements, 1999. http://www.icru.org/home/reports/prescribing-recording-and-reporting-photon-beam-therapy-report-62
6. Allibhai Z, Taremi M, Bezjak A, Brade A, Hope AJ, Sun A, Cho BC. The impact of tumor size on outcomes after stereotactic body radiation therapy for medically inoperable early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2013 Dec 1;87(5):1064-70.
7. Nieder C, Grosu AL, Gaspar LE. Stereotactic radiosurgery (SRS) for brain metastases: a systematic review. Radiat Oncol. 2014 Jul 12;9:155.
8. Roesch, J., Andratschke, N., & Guckenberger, M. (2014). SBRT in operable early stage lung cancer patients. Translational Lung Cancer Research, 3(4), 212–224.
9. Onishi H, Kuriyama K, Komiyama T, Tanaka S, Sano N, Aikawa Y, Tateda Y, Araki T, Ikenaga S, Uematsu M. A new irradiation system for lung cancer combining linear accelerator, computed tomography, patient self-breath-holding, and patient-directed beam-control without respiratory monitoring devices. Int J Radiat Oncol Biol Phys. 2003 May 1;56(1):14-20.
10. Negoro Y, Nagata Y, Aoki T, Mizowaki T, Araki N, Takayama K, Kokubo M, Yano S, Koga S, Sasai K, Shibamoto Y, Hiraoka M. The effectiveness of an immobilization device in conformal radiotherapy for lung tumor: reduction of respiratory tumor movement and evaluation of the daily setup accuracy.Int J Radiat Oncol Biol Phys. 2001 Jul 15; 50(4):889-98.
11. Uematsu M, Shioda A, Suda A, Fukui T, Ozeki Y, Hama Y, Wong JR, Kusano S. Computed tomography-guided frameless stereotactic radiotherapy for stage I non-small cell lung cancer: a 5-year experience. Int J Radiat Oncol Biol Phys. 2001 Nov 1; 51(3):666-70.
12. Coon D, Gokhale AS, Burton SA, et al. Fractionated stereotactic body radiation therapy in the treatment of primary, recurrent, and metastatic lung tumors: the role of position emission tomography/computed tomography-based treatment planning. Clin Lung Cancer. 2008; 9:217–21.
13. Tanyi JA, Summers PA, McCracken CL, Chen Y, Ku L-C, Fuss M. Implications of a high-definition multileaf collimator (HD-MLC) on treatment planning techniques for stereotactic body radiation therapy (SBRT): a planning study. Radiation Oncology (London, England). 2009; 4:22.
14. Lim do H, Yi BY, Mirmiran A, Dhople A, Suntharalingam M, D'Souza WD. Optimal beam arrangement for stereotactic body radiation therapy delivery in lung tumors. Acta Oncol. 2010; 49(2):219-24.
15. Teoh M, Clark CH, Wood K, Whitaker S, Nisbet A. Volumetric modulated arc therapy: a review of current literature and clinical use in practice. The British Journal of Radiology. 2011; 84(1007):967-996.
16. Webb S, McQuaid D. Some considerations concerning volume-modulated arc therapy: a stepping stone towards a general theory. Phys. Med. Biol.2009; 54; (14) 43-45.
17. Sterzing F, Kalz J, Sroka-Perez G, Schubert K, Bischof M, Roder F, Debus J, Herfarth K. Megavoltage CT in helical tomotherapy - clinical advantages and limitations of special physical characteristics. Technol Cancer Res Treat. 2009 Oct; 8(5):343-52.
18. Li J, Galvin J, Harrison A, Timmerman R, Yu Y, Xiao Y. Dosimetric verification using monte carlo calculations for tissue heterogeneity-corrected conformal treatment plans following RTOG 0813 dosimetric criteria for lung cancer stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2012 Oct 1; 84(2):508-13.
19. Papiez L, Timmerman R, DesRosiers C, Randall M. Extracranial stereotactic radioablation: physical principles. Acta Oncol. 2003; 42(8):882-94.
20. Rana S, Rogers K, Pokharel S, Cheng C. Evaluation of Acuros XB algorithm based on RTOG 0813 dosimetric criteria for SBRT lung treatment with RapidArc. J Appl Clin Med Phys. 2014 Jan 6; 15(1):4474.
21. Shaw E, Kline R, Gillin M, Souhami L, Hirschfeld A, Dinapoli R, Martin L. Radiation Therapy Oncology Group: radiosurgery quality assurance guidelines Int J Radiat Oncol Biol Phys. 1993 Dec 1; 27(5):1231-9.
22. Kataria T, Sharma K, Subramani V, Karrthick KP, Bisht SS. Homogeneity Index: An objective tool for assessment of conformal radiation treatments. Journal of Medical Physics/Association of Medical Physicists of India. 2012; 37(4):207-213.
23. Paddick I, Lippitz B.A simple dose gradient measurement tool to complement the conformity index J Neurosurg. 2006 Dec; 105 Suppl:194-201.
24. G.G. Steel, T.J. McMillan, J.H. Peacock. The 5 Rs of radiobiology. Int J Radiat Biol, 56 (6) (1989), pp. 1045–1048.
25. Eric J. Hall and Amato J. Giaccia. Oxygen Effect and Reoxygenation. In Radiobiology for the radiologist, Lippincott Williams & Wilkins, 6th Ed., 2006.
26. Tipton KN, Sullivan N, Bruening W, et al. Stereotactic Body Radiation Therapy: Agency for Healthcare Research and Quality (US); 2011 May. (Comparative Effectiveness Technical Briefs, No. 6.) Available from: http://www.ncbi.nlm.nih.gov/books/NBK55723/
27. Shibamoto Y, Miyakawa A, Iwata H, Otsuka S. Radiobiology of SBRT. In Stereotactic Body Radiation Therapy: Principles and Practices, 2015; 11-25, Springer Japan
28. Radiation Biology: A Handbook for Teachers and Students. Training Courses Serie 42. Vienna 2010. www-pub.iaea.org/MTCD/publications/PDF/TCS-42_web.pdf
29. Chang W. Song, Heonjoo Park, Robert J. Griffin, and Seymour H. Levitt. Radiobiology of Stereotactic Radiosurgery and Stereotactic Body Radiation Therapy. In Technical Basis of Radiation Therapy, Medical Radiology. Radiation Oncology. Springer-Verlag Berlin Heidelberg 2012.
30. Pawlik TM, Keyomarsi K. Role of cell cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol Biol Phys. 2004 Jul 15; 59(4):928-42.
31. Withers HR. Cell cycle redistribution as a factor in multifraction irradiation. Radiology. 1975 Jan; 114(1):199-202.
32. Tubiana M, Dutreix J, Wambersie A. The effects of radiation on tumours. The biological basis of radiotherapy. In Introduction To Radiobiology. Taylor & Francis, 1990.
33. Royal College of Radiologists. The timely delivery of radical radiotherapy: standards and guidelines for the management of unscheduled treatment interruptions. 2008. (Third edition). https://www.rcr.ac.uk/docs/oncology/pdf/BFCO(08)6_Interruptions.pdf. [Online] 2008.
34. J Deacon, M.J Peckham, G.G Steel. The radioresponsiveness of human tumours and the initial slope of the cell survival curve. Radiother Oncol, 2 (1984), pp. 317–323.
35. F.B. Geara, L.J. Peters, K.K. Ang, et al. Prospective comparison of in vitro normal cell radiosensitivity and normal tissue reactions in radiotherapy patients. Int J Radiat Oncol Biol Phys, 27 (1993), pp. 1173–1179.
36. C.M.L West, S.E Davidson, S.A Roberts, et al. The independence of intrinsic radiosensitivity as a prognostic factor for patient response to radiotherapy of carcinoma of the cervix. Br J Cancer, 76 (1997), pp. 1184–1190.
37. Björk-Eriksson T1, West C, Karlsson E, Mercke C. Tumor radiosensitivity (SF2) is a prognostic factor for local control in head and neck cancers. Int J Radiat Oncol Biol Phys. 2000 Jan 1; 46(1):13-9.
38. C.E. Redon, J.S. Dickey, W.M. Bonner, et al. γ-H2AX as a biomarker of DNA damage induced by ionizing radiation in human peripheral blood lymphocytes and artificial skin. Adv Space Res, 43 (2009), pp. 1171–1178.
39. S. Malone, G.P. Raaphorst, R. Gray, et al. Enhanced in vitro radiosensitivity of skin fibroblasts in two patients developing brain necrosis following AVM radiosurgery: a new risk factor with potential for a predictive assay. Int J Radiat Oncol Biol Phys, 47 (2000), pp. 185–189.
40. G.P. Raaphorst, S. Malone, G. Alsbeih, et al. Skin fibroblasts in vitro radiosensitivity can predict for late complications following AVM radiosurgery. Radiother Oncol, 64 (2002), pp. 153–156.
41. S. Malone, J. Szanto, G. Alsbeith, et al. Radiation sensitivity testing and late neurological complications following radiosurgery for AVM: the use of SF2 from fibroblasts as a predictive factor. Cancer Radiother, 7 (2003), pp. 225–230.
42. Adams G, Martin OA, Roos DE, Lobachevsky PN, Potter AE, Zacest AC, Bezak E, Bonner WM, Martin RF, Leong T. Enhanced intrinsic radiosensitivity after treatment with stereotactic radiosurgery for an acoustic neuroma.Radiother Oncol. 2012 Jun;103(3):410-4. doi: 10.1016/j.radonc.2012.03.011. Epub 2012 May 3.
43. D. Kondziolka, L.D. Lunsford, M.R. McLaughlin, et al. Long-term outcomes after radiosurgery for acoustic neuromas. NEJM, 339 (1998), pp. 1426–1433.
44. Kim MS, Kim W, Park IH, Kim HJ, Lee E, Jung JH, Cho LC, Song CW. Radiobiological mechanisms of stereotactic body radiation therapy and stereotactic radiation surgery. Radiat Oncol J. 2015 Dec;33(4):265-75. doi: 10.3857/roj.2015.33.4.265. Epub 2015 Dec 30.
45. Song CW, Park I, Cho LC, et al. Is indirect cell death involved in response of tumors to stereotactic radiosurgery and stereotactic body radiation therapy? Int J Radiat Oncol Biol Phys. 2014; 89:924–925.
46. Tipton KN, Sullivan N, Bruening W, et al. Stereotactic Body Radiation Therapy [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2011 May. (Comparative Effectiveness Technical Briefs, No. 6.) Results. Available from: http://www.ncbi.nlm.nih.gov/books/NBK55731
47. Harada T, Shirato H, Ogura S, Oizumi S, Yamazaki K, Shimizu S, Onimaru R, Miyasaka K, Nishimura M, Dosaka-Akita H. Real-time tumor-tracking radiation therapy for lung carcinoma by the aid of insertion of a gold marker using bronchofiberscopy. Cancer. 2002 Oct 15; 95(8):1720-7.
48. Ionascu D, Jiang SB, Nishioka S, Shirato H, Berbeco RI. Internal-external correlation investigations of respiratory induced motion of lung tumors. Med Phys. 2007 Oct; 34(10):3893-903.
49. Neicu T, Berbeco R, Wolfgang J, Jiang SB. Synchronized moving aperture radiation therapy (SMART): improvement of breathing pattern reproducibility using respiratory coaching. Phys Med Biol 2006; 51: 617–36.
50. Gaya A M, Mahadevan A. Introduction to Stereotactic Body Radiotherapy in Stereotactic Body Radiotherapy. A Practical Guide, Springer (2015): 1-18.