We present a short review about the main symptoms encountered in lung cancer, namely dyspnea, cough and hemoptysis. A particular aspect is represented by the rales of patients in the state of end of life. The pharmacological means of treatment are described. Following the most recent studies, classical drugs like opioids and benzodiazepines have been reevaluated as useful and indicated. There are also presented some nonpharmacological means.
Symptoms control in patients with advanced lung cancer
Controlul simptomelor pacienţilor cu cancer pulmonar avansat
First published: 25 martie 2022
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/OnHe.58.1.2022.6226
Abstract
Rezumat
Prezentăm un scurt review despre principalele simptome întâlnite în cancerul pulmonar, şi anume dispneea, tusea şi hemoptizia. Un aspect particular îl reprezintă şi ralurile pacienţilor din starea de sfârşit al vieţii. Sunt prezentate mijloacele farmacologice de tratament, cum sunt opioizii şi benzodiazepinele, care, în urma studiilor mai recente, au fost reevaluate ca având utilitate şi fiind indicate. De asemenea, sunt prezentate şi câteva mijloace nefarmacologice.
Introduction
Millions of people in the world are distressed by respiratory symptoms, resulting from the highly prevalent lung cancer and pulmonary metastases(1). Respiratory symptoms such as cough and dyspnea are common in patients with advancing and incurable disease, giving rise to varying degrees of respiratory distress which adversely affects the patient’s quality of life. Other main respiratory problems are death rales, acute suffocation and hemoptoea.
In recent years, there has been a significant growth into the palliation of respiratory symptoms, leading to practical ways of giving relief in hospices, hospitals and at home.
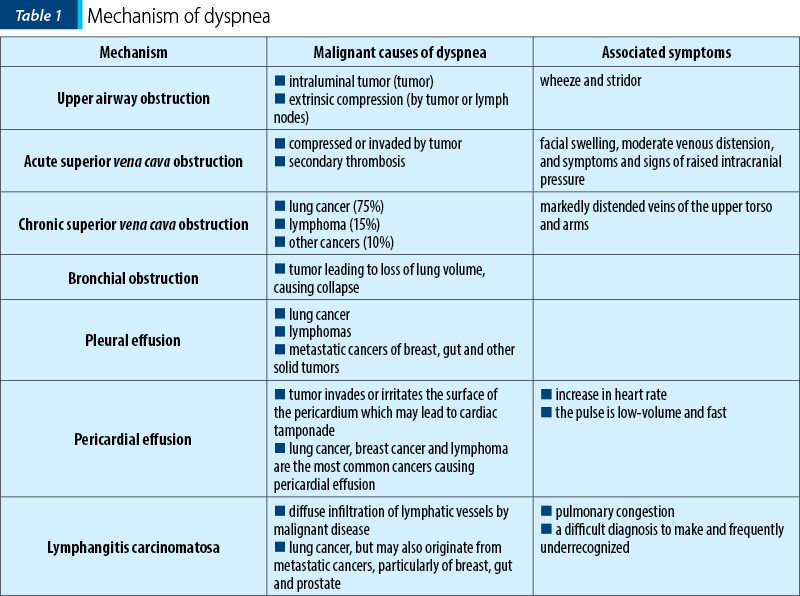
DYSPNEA
Dyspnea is an unpleasant sensation of being unable to breathe easily and causes anxiety in both patients and their carers. Dyspnea is a subjective experience of difficult, labored and uncomfortable breathing which may be described as: shortness of breath, a smothering feeling, inability to get enough air or suffocation. Dyspnea is one of the most commonly reported symptoms in lung cancer, with an incidence of 15% at diagnosis and 65% at the end of disease evolution(1).
The prevalence of dyspnea varies with the localization of primary malignancy, the stage of disease and with other factors. During the last days of life, this symptom changes little in prevalence, dyspnea becoming more frequent.
For patients with non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC), the prevalence rate is as high as 85%.
Etiology
The causes of dyspnea in advanced cancer are multiple and they can be classified by four approaches:
a) local or systemic causes;
b) relationship with tumor (malignant, paramalignant, non-malignant);
c) according to physiological impairment
-
lung function patern (obstructive, restrictive, mixed);
-
oxygen saturation (hypoxic, non-hypoxic).
The identification of dyspnea causes guides its treatment, but this is often limited by the altered performance status and the bad prognosis(2).
Diagnosis
The level of dyspnea experienced is not predicted by normal tests of respiratory function and these tests are not useful in assessing the need for treatment or for ongoing monitoring. The subjective nature of dyspnea requires assessment based on the patient’s description of their experience(4). Patients often have several different underlying factors that lead to the development of dyspnea.
The elucidation of all the underlying causes relies on good history, detailed physical examination and carefully selected investigations. History findings in cancer patients with dyspnea are: smoking, occupational exposure, drug history, past anticancer treatment, concomitant medical illness, associated respiratory symptoms and special patterns of dyspnea(1). Tachypnea (rapid breathing) often accompanies dyspnea. If panic and anxiety are present, they lead to a central increase in the rate of breathing which further increases the feeling of breathlessness and anxiety. A vicious circle is started which is then difficult to break. A prospective study of 100 terminally ill cancer patients (49 patients with lung cancer) observed that dyspnea, measured on the visual analogue scale, was significantly associated with anxiety (p=0.001)(2). Increased anxiety has been connected with worse dyspnea in patients with cancer. One study on 120 patients with stages I through IV lung cancer observed no difference in dyspnea based on cancer stage, cell type or performance status. However, pain and anxiety scores were higher in patients with high dyspnea scores(2).
The investigations should be carefully selected to guide the specific treatment. The most important investigations are: hemoglobin level, oxygen saturation (oximetry – a noninvasive method to identify the hypoxic patients) and chest radiograph, which may be informative in defining specific dyspnea syndromes.
Dyspnea measuring scales
There are scales measuring multiple symptoms including dyspnea and scales for measuring the single symptom of dyspnea. In the first group, we can mention: the Edmonton Symptom Assessment System (ESAS), for nine cancer-related symptoms; the Suport Team Assessment Schedule (STAS), a five- or seven-point scale that can measure dyspnea in terms of intensity, frequency and its interference with activity. In order to measure the single symptom of dyspnea, they can use VAS, a verbal rating scale, or the Likert-type scale. Recently, the Cancer Dyspnea Scale (CDS) has been validated in lung cancer patients for measuring dyspnea(3).
Treatment
Where appropriate, the treatment of any underlying cause, such as anemia, infection or pulmonary embolus, should be undertaken and some patients may benefit from specific anticancer treatment. There is evidence that patients with no apparent lung disease can suffer from breathlessness, probably as a result of respiratory muscle weakness due to severe cachexia. Therefore, the majority of patients will require symptomatic treatment based on the clinical characteristics of their breathlessness.
The accurate diagnosis of the cause of the dyspnea is required if the underlying cause is to be treated, but multiple causes of dyspnea are often present and should generally be treated concurrently(3).
The treatments of underlying physical causes of malignant dyspnea are presented in Table 2.
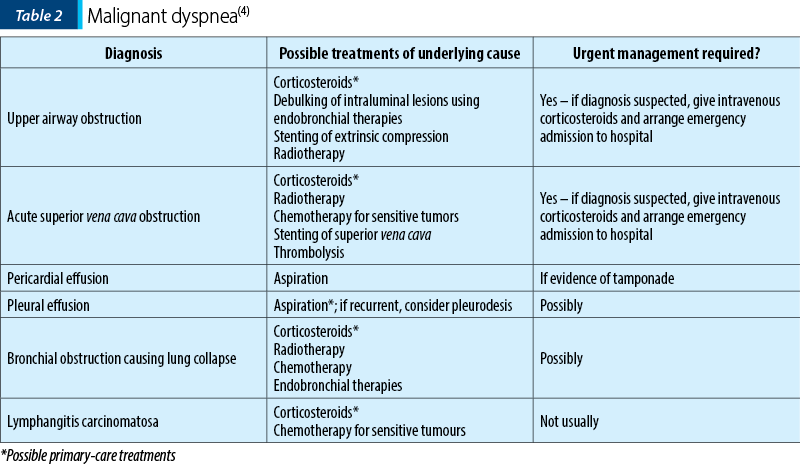
The pharmacologic treatments for dyspnea caused by lung cancer included oxygen, bronchodilators, corticosteroids, antibiotics and opioids. One retrospective study at a medical center specialized in cancer assessed the resource utilization associated with the management of dyspnea caused by lung cancer in 45 patients. The most common therapies administered in the emergency department were oxygen (31%), beta-2 agonists (19%), antibiotics (12%) and opioids (11%). Supplemental oxygen is the most commonly prescribed therapy to relieve dyspnea, but a limited number of studies have shown the beneficial effects of supplemental oxygen therapy. Oxygen has been shown to be effective in reducing dyspnea in patients who are hypoxic and dyspneic at rest. The therapeutic value of oxygen therapy in other groups of patients with dyspnea is unclear. It is uncertain whether oxygen is better than air for relieving dyspnea in patients with advanced cancer(4). A prospective, double-blind, crossover trial assessed the effects of supplemental oxygen on the intensity of dyspnea in 14 patients with advanced cancer. The results showed that 12 patients consistently preferred oxygen to air and the patients reported little or no benefit from air compared to moderate to much benefit from oxygen. Irrespective of the oxygenation status, supplemental oxygen therapy should be considered if patients with lung cancer have dyspnea.
The patients with a history of reversible airway disease, chronic obstructive pulmonary disease (COPD) or with symptoms of wheezing may benefit from regular bronchodilators. A prospective study of 100 terminally ill cancer patients (49 with lung cancer) observed that the potentially correctable causes of dyspnea included bronchospasm (in 52%) and hypoxia (in 40%). It is important to ensure that bronchodilator therapy is optimized if the patient has obstructive airways disease.
The role of systemic corticosteroids is limited for relieving dyspnea from lung cancer, but they are commonly used even though there are no controlled studies to support their use. As is the case with bronchodilator therapy, patients with obstructive airways disease may benefit from treatment with systemic corticosteroids to decrease mucus production and inflammatory changes in the airway mucosa. In one study, 50% of patients had an element of bronchospasm to their dyspnea. All patients may be given a trial of an oral steroid, either for an anti-inflammatory effect or to reduce peritumor edema, unless a contraindication exists(4). Because dexamethasone is used empirically for dyspnea, it is important to taper to the minimum the effective dose that controls the symptoms. There is little evidence to guide the best starting dose of oral dexamethasone, a reported starting dosage of dexamethasone being 8-12 mg/day.
If tachypnea is also a central feature of the respiratory difficulties, opioids are useful to decrease central respiratory drive. Smaller doses and dose increments of opioids than those used for pain relief are titrated against subjective response(4). It is not clear if all opioids are equally efficacious in decreasing dyspnea perception in patients with lung cancer. In a study of 104 patients with lung cancer, opioids administered to treat pain did not decrease dyspnea. The relation between opioids and respiration is not simple; if used inappropriately, opioids can induse respiratory depression, which is determined by pathophysiology, prior exposure to opioids, rate and route of dose titration, and coexisting pathology. However, low doses of oral opioids can improve breathlessness, sometimes dramatically, although the precise mechanism of action is unknown. The dose of opioids can be titrated in the same way as when used for pain control, but lower doses and smaller increments should be used. In patients not previously exposed to opioids, as little as 2.5 mg of oral morphine every four hours may be sufficient. A dose of 5-10 mg regularly every four hours, and as required, should be used for patients who have previously been taking a weak opioid – e.g., codeine. If the patient is already on regular morphine, increase the dose by 30-50% every 2-3 days, until the symptoms are controlled or the adverse effects prevent further dose increases(4).
A new study found that pharmacologic interventions were not associated with more effectiveness than placebo for the management of breathlessness in patients with advanced cancer. In this respect, practitioners may need to reevaluate the role of the pharmacologic agents, such as opioids and anxiolytics, in the treatment of dyspnea for patients with advanced cancer, taking into consideration patient-related factors(5).
Benzodiazepines are effective in low doses, particularly for patients whose anxiety augments the dyspnea, although the benefit in patients with no apparent anxiety can also occur, probably due to sedation and muscle relaxation. Lorazepam 0.5-2 mg given sublingually can be useful in acute attacks. If the regular treatment is required, diazepam 5 mg daily is started and the dose slowly titrated upwards to obtain the maximum response with minimum sedation.
Breathlessness is often an emotion-loaded experience associated with fear, anxiety, helplessness, panic or depression. The optimal control of dyspnea is achieved when drug treatment is given in conjunction with physiotherapy, counseling and the provision of practical aids for daily living. Patients may have feelings of impending death during the acute dyspneic attack. Patients should be informed about the measures they can initiate and which allow them to regain control: stop (try to stay calm), purse lips, drop (relax shoulders, back, neck and arms) and flop (concentrate on breathing out slowly).
A multicenter randomized controlled trial was conducted on 119 patients with lung cancer or mesothelioma who had completed the first-line treatment for their diseases and reported dyspnea. The patients in the intervention group attended a weekly nursing clinic for up to 8 weeks. There were used various strategies: breathing control, activity pacing, relaxation techniques and psychosocial support in addition to the standard treatment available for dyspnea. The group assigned to intervention by nurses improved significantly at 8 weeks regarding breathlessness, performance status and physical and emotional status, compared to the control group(2). Control breathing techniques include: positioning, pursed-lip breathing (PLB), breathing exercises and coordinated breathing training. Pacing of breathing with activity, energy conservation techniques and home modification can maintain the patient’s basic activities of daily living.
Unfortunately, trials for benzodiazepines have failed to provide evidence of benefit(6).
COUGH
Cough is a normal but complex physiological mechanism that protects the airways and lungs by removing mucus and foreign matter from the larynx, trachea and bronchi, and is under both voluntary and involuntary control. The pathological cough is common in malignant and non-malignant disease. Breathlessness can trigger cough and vice versa. Persistent cough can also precipitate vomiting, exhaustion, chest or abdominal pain, rib fracture, syncope and insomnia. Cough has a prevalence of 47-86% in lung cancer and 23-37% in general cancer patients. Cough of moderate to severe intensity occurred in 13% of general cancer patients and in 17-48% of lung cancer patients. Few studies quantitatively examined the distress caused by cough in cancer patients. In one series of 240 cancer patients, of which 21.3% had lung cancer, cough was present in 33%. Among all patients, 13% had moderate to severe cough and 18% of all patients suffered from severe distress from cough(1). In lung cancer, cough is a frequent and distressing symptom, which can be dry or associated with sputum production. Among the initial symptoms of lung cancer, cough is present in less than 65% of patients and productive cough is present in more than 25% of patients.
Etiology
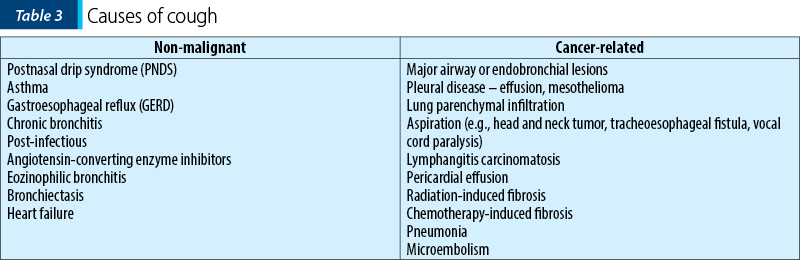
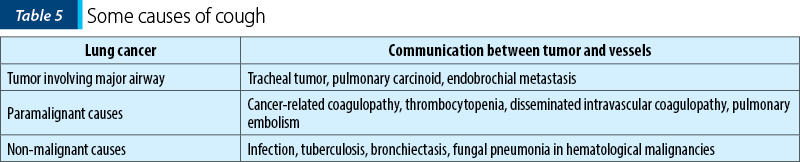
Chronic cough is due to multiple causes in the general population. In cancer, it is likely that multiple causes – and hence multiple mechanisms – are responsible. The causes of cough in cancer are presented in Table 3.
Classification of types of coughs: productive cough in the patient who is able to cough effectively, productive cough in the patient who is not able to cough effectively, and non-productive cough.
Treatment
In the general population, treating cough is highly successful if the underlying cause is identified. In cancer, the search for a specific cause may be limited by the burden of investigations, so the treatment of cough can be categorized into: specific treatment of the underlying causes, enhancing effectiveness of cough when indicated, and suppression of cough. The methods of specific treatment of cough are presented in Table 4.
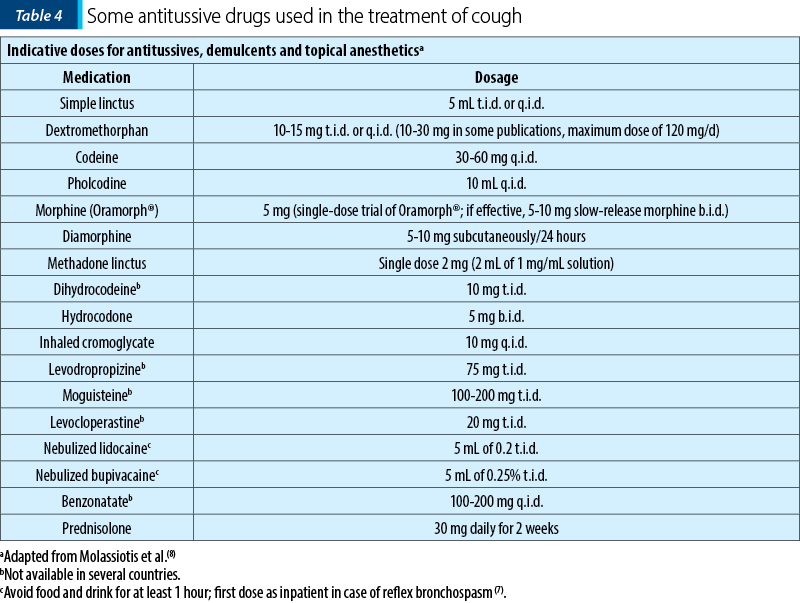
Opioids are the best cough suppressants in patients with lung cancer, especially in advanced stages, when standard non-opioid cough suppressants may not control the cough. Codeine is the most widely used opioid. A double-blind, randomized controlled trial regarding the treatment of nonproductive cough was performed in 140 adults with primary lung cancer or metastatic cancer of the lungs. The therapeutic efficacy and the tolerability of a seven-day treatment with levodropropizine drops (75 mg t.i.d.) were evaluated in comparison with dihydrocodeine drops (10 mg t.i.d.). The efficacy was assessed on the basis of cough severity scores, the number of night awakenings due to cough, and the overall estimate of antitussive efficacy. Subjective cough severity was significantly reduced during the treatment with levodropropizine and dihydrocodeine, the antitussive effect and its time profile being similar for both drugs. Also, according to the investigator’s evaluation, both levodropropizine and dihydrocodeine treatment produced a significant decrease in cough severity. Concurrently with the relief of cough, the number of night awakenings was decreased significantly by both drugs, with no difference between the two treatments. However, the percentage of patients experiencing somnolence in the group receiving levodropropizine (8%) was significantly lower as compared with that of the dihydrocodeine group (22%). These results confirm the antitussive effectiveness of levodropropizine and suggest a more favorable benefit/risk profile when compared to dihydrocodeine. However, levodropropizine is not available for use in the United States of America.
Non-opioid cough suppressants may work in a small group of patients with advanced lung cancer.
Occasionally, even opioid-resistant cough may respond to agents such as the peripherally acting nonopioid drug benzonatate.
Bronchospasm can cause or contribute to cough. If the patient with lung cancer has also underlying bronchospastic obstructive airways disease, then standard bronchodilator therapy may help alleviate the cough. One study tested the role of inhaled sodium cromoglycate in 20 patients with NSCLC and cough resistant to conventional treatment. The patients were randomized to receive, in a double-blind trial, either inhaled sodium cromoglycate or placebo. The results showed that inhaled sodium cromoglycate reduced cough in all patients with NSCLC.
There are no studies on steroids specifically for cough in lung cancer. If cough is caused by radiation-induced lung problems, then high-dose corticosteroid therapy may relieve a significant degree of cough.
There are no studies on the role of inhaled lidocaine on cough in patients with lung cancer(8).
Bronchoscopy methods to palliate dyspnea and cough
Most patients with dyspnea caused by central airway obstruction also complain of cough. The severity of dyspnea is dependent on the extent of luminal involvement of the airway and the presence or absence of underlying conditions such as COPD, cardiac failure, loss of lung tissue from previous lung surgery etc.
Extraluminal tumor compression of the major airways, intraluminal tumor growth, or a combination of both, can cause central airway obstruction. Perhaps the most important aspect of managing these patients is to first determine the anatomic type of airway involvement. However, the single most important diagnostic procedure is bronchoscopy. It will also help determining if bronchoscopy therapy is feasible. Almost all endobronchial therapies are palliative in patients with lung cancer involving the major airways. The relief of cough is more troublesome because none of the therapies discussed below will totally eradicate the tumor. The degree of dyspnea and respiratory distress should dictate the appropriate mode of endobronchial therapy. The currently available methods of bronchoscopy therapy include debulking of intraluminal tumor growth (usually with rigid bronchoscopy), balloon dilatation, laser therapy, electrocautery, cryotherapy, argon plasma coagulation (APC), endobronchial irradiation, or intraluminal stent placement.
All of these therapeutic techniques will provide significant relief of dyspnea and cough in the majority of patients(9).
HEMOPTYISIS
Hemoptysis is defined as coughing up of blood. The most common causes are chronic bronchitis, cancer and tuberculosis.
Incidence: 20% of patients with lung cancer will have a hemoptysis in their evolution, lung being the most frequent causes among all cancers. Aproximatively 3% of patients with lung cancer had massive terminal hemoptysis. In 18% of cases, massive hemoptysis was associated with squamous cell carcinoma. The causes of bleeding have changed in the last decades, therefore the frequency of bleeding caused by cancer has increased while the bleeding caused by tuberculosis has decreased(1).
Direct causes of bleeding and prognosis
The predominant source of bleeding is the bronchial arterial system affected by superficial mucosal inflammation or erosion. Massive hemoptysis (MH) represents the expectoration of at least 100 to 600 mL of blood in 24 hours. Blood clot is related to the respiratory insufficiency caused by airway obstruction. The prognosis in MH is very poor; the mortality may be 59% in patients with lung cancer.
Diagnosis and differential diagnosis
The clinical manifestations of the primary disease dominate the clinical presentation. The evaluation is done by medical history, physical examination and specific investigation for underlying disease (radiography, bronchoscopy, laboratory tests.
The differential diagnosis must be made with:
-
bleeding from the upper airway (sinusitis, nasal polyps, laryngeal and nasopharyngeal neoplasms);
-
bleeding as a result of aspiration from the gastrointestinal tract (nausea, vomiting);
-
pseudohemoptysis – pigmentation produced by microbes like Serratia marcescens or by medication.
Management
The surgical resection of the bleeding lobe when the hemoptysis is a symptom at the moment of lung cancer diagnosis (the patient must be amenable to surgery with curative intent). The management of MH is usually by endotracheal intubation, the single lumen tube being the best solution. Bronchoscopy is useful to identify the source of bleeding. The endobronchial treatment consists in continuous suctioning to collapse the segment. This management is used when only the location of bleeding is found, but not the direct source. The epinephrine solution (1/10,000) is useful to be instilated. Balloons may be also used to control by tamponade the bleeding. Nd:YAG laser can stop the bleeding by photocoagulation. Electrocautery has some efficiency (60%). For endobronchial cancer visualized by bronchial bronchoscopy, artery embolization is beneficial. For unresectable lung cancer diagnos by bronchoscopy, external beam radiotherapy must be applied.
Palliation of hemoptysis:
-
Single lumen endotracheal intubation.
-
Bronchoscopy to identify the source of bleeding.
-
Bronchial tamponade by aspiration.
-
Bronchial artery embolization.
-
External beam radiation.
-
Pharmacological approach
- tranexamic acid, 1000 to 1500 mg x 3
- prednisone, 40 to 60 mg x 1, or dexamethasone, 6 to 9 mg x 1, with dose tapering according to response(10-12).
DEATH RATTLE
Death rales (death rattle) occur in the terminal stage of cancer or other diseases (progressive neuromuscular disorders etc.).
Prognostic: predicting of short-term dying is useful but not infallible.
In the final hour, generally the patients are semiconscious or unconscious, they are unable to swallow saliva and unable to cough the mucus from the trachea. Breathing with partial obstruction caused by these secretions in central airways or glottic area gives rise to a noisy respiration which was called “death rattle”. These rales are better detected by direct palpation of the chest wall for vibrations (anterior chest over the trachea). Other symptoms of this terminal syndrome are: inappetence, little or any intake of food or fluids, poor communication (rarely speaking), cough, increase or irregular respiratory rate and sometimes Cheyne-Stokes respirations. The pulse may be strong initially, but becomes thready or not palpable.
The temperature could be higher due to vasodilatation (eventually sepsis). The prevention with an anticholinergic (hyoscine), given as single parenteral dose or by continuous infusion, along with the reposition of the patient or suction with a soft catheter could be useful(13).
Conflict of interests: The author declares no conflict of interests.
Bibliografie
-
Dyspnea. Mechanisms, assessment, and management: a consensus statement. American Thoracic Society. Am J Respir Crit Care Med. 1999 Jan;159(1):321-40.
-
Doyle D, Hanks G, Cherny N, Sir Calman K. Oxford Textbook of Palliative Medicine. Third Edition, Oxford University Press ,2004.
-
https://www.healthline.com/health/dyspnea
-
Kvale P, Simoff M, Prakash U. Palliative Care. Chest. 2003 Jan 1;284S–311.
-
Feliciano JL, Waldfogel JM, Sharma R, Zhang A, Gupta A, Sedhom R, Day J, Bass EB, Dy SM. Pharmacologic Interventions for Breathlessness in Patients with Advanced Cancer: A Systematic Review and Meta-analysis. JAMA Netw Open. 2021 Feb 1;4(2):e2037632.
-
Barnes H, Natasha Smallwood N. Pharmacological Interventions for Breathlessness – Absence of Effect or Absence of Evidence?, JAMA Netw Open. 2021;4(2):e210262. doi:10.1001/jamanetworkopen.2021.0262
-
Simon ST, Higginson IJ, Booth S, Harding R, Weingartner V, Bausewein C. Benzodiazepines for the relief of breathlessness in advanced malignant and non-malignant diseases in adults. Cochrane Database Syst Rev. 2016;10:CD007354.
-
Molassiotis A, Smith JA, Mazzone P, Blackhall F, Irwin RS; CHEST Expert Cough Panel. Symptomatic Treatment of Cough Among Adult Patients with Lung Cancer: CHEST Guideline and Expert Panel Report. CHEST. 2017 Apr;151(4):861-874.
-
https://www.cochrane.org/CD007881/SYMPT_interventions-cough-patients-cancer
-
https://www.uptodate.com/contents/endobronchial-brachytherapy
-
Gershman E, Guthrie R, Swiatek K, Shojaee S. Management of hemoptysis in patients with lung cancer. Ann Transl Med. 2019 Aug;7(15):358.
-
Jusyk K, Siemińska A, Jassem E. Hemoptysis – causes, diagnosis and treatment. Medycyna Paliatywna w Praktyce. 2017;11(4):166-173.
-
Twomey S, Dowling M. Management of death rattle at end of life. Br J Nurs. 2013 Jan 24-Feb 13;22(2):81-5.
Articole din ediţiile anterioare
Breviar terapeutic al cancerului pulmonar fără celule mici, din perspectiva oncologiei medicale
Acest ghid sumarizează recomandările Societăţii Americane de Oncologie Clinică şi prezintă într-o manieră schematică principalele modalităţi de abo...
Abordarea chirurgicala a unui nodul pulmonar solitar la un pacient cu o neoplazie in antecedente
Un nodul pulmonar solitar reprezintă o opacitate radiologică, mai mică de 3 centimetri, înconjurată de parenechim pulmonar normal, fără alte modifi...
Radiomica şi radiogenomica în managementul cancerului bronhopulmonar – o scurtă recenzie
Cancerul bronhopulmonar este principala cauză a mortalităţii prin cancer, diagnosticul tardiv fiind una dintre cauzele asociate cu decesul şi cu ...
Importanţa examenului imunohistochimic în stabilirea prognosticului cancerului pulmonar fără celule mici
Cancerul pulmonar este prima cauză de deces prin cancer la nivel european şi mondial. Aproximativ 80% din cancerele pulmonare sunt reprezentate de ...