Prematurity still represents a major cause for many deaths in the neonatal period and through the age of 5 years old, all over the world, although huge progress has been made in terms of treating and preventing their main morbidity causes. The prognosis of preterm infants is marked by the occurrence of necrotizing enterocolitis (NEC) and late-onset sepsis (LOS). Being born preterm alters the process of bacterial colonization as a consequence of a multifactorial etiology, such as immaturity of the gut environment, exposure to antibiotics, and supplemental feeding with formula. In order to reach the main health benefits of the probiotic treatment, mainly NEC prevention, this should be initiated before and during the most vulnerable period, which is not immediately after birth. Although using probiotics during the neonatal period is sustained by researchers with enthusiasm, the preterm infant has a particular gut environment, and some interactions might not have the proposed benefits. Anyway, the actual recommendations advise for using some specific strains, and recommend against the use of others. Further randomized controlled trials exploring probiotic supplementation in clinical outcomes of preterms are still needed.
Dezbateri curente: tendinţe în utilizarea probioticelor la nou-născuţii prematuri
Current debate: the trend in probiotic use in preterm infants
First published: 16 decembrie 2020
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/Pedi.60.4.2020.4069
Abstract
Rezumat
Prematuritatea continuă să reprezinte o cauză majoră de morbiditate şi mortalitate în perioada neonatală, determinând prognosticul stării de sănătate până la vârsta de 5 ani în întreaga lume, deşi s-au înregistrat progrese importante în tratarea şi prevenirea principalelor cauze ale acesteia. Prognosticul copiilor prematuri este marcat de apariţia enterocolitei necrozante (ECN) şi a sepsisului cu debut tardiv. Naşterea prematură modifică procesul de colonizare bacteriană, fiind o consecinţă a unei etiologii multifactoriale, printre care se numără imaturitatea climatului intestinal, expunerea la antibiotice şi alimentaţia artificială. Pentru a atinge principalele beneficii pentru sănătate ale tratamentului cu probiotice, cu scopul de preveni instalarea ECN, acesta trebuie iniţiat înainte de perioada cea mai vulnerabilă, dar şi în timpul acesteia, în momentul în care nou-născuţii prematuri au riscul cel mai ridicat de a dezvolta sepsis sau ECN. Deşi sunt suficiente premise pentru utilizarea probioticelor în perioada neonatală, nou-născutul prematur prezintă un climat intestinal special, iar interacţiunile complexe dintre microbiotă, alimentaţie şi dezvoltarea infecţiilor ar putea să nu aibă întotdeauna beneficiile propuse. Ghidul actual pentru utilizarea probioticelor în managementul prematurităţii face referire la utilizarea anumitor tulpini, la necesitatea elementelor de siguranţă a speciilor utilizare, susţinând în continuare necesitatea unor studii controlate randomizate, care să ofere o uniformitate a datelor.
Introduction
Prematurity still represents a major cause for many deaths around the neonatal period and through the age of 5 years old, all over the world, although huge progress has been made in terms of treating and preventing their main morbidity causes(1). The prognosis of preterm infants is marked by the occurrence of necrotizing enterocolitis (NEC) and the late-onset sepsis (LOS)(2). Thus, there is no surprise that important researches were performed in terms of preventive strategies of these comorbidities, trying to reduce the incidence for both NEC and LOS. One of the main interventions is considered to be the probiotics administration, as a so-called “golden age” of neonatal medicine, because a growing body of evidence is directed towards using probiotics in improving short- and long-term consequences of prematurity(3). Nevertheless, the general consensus is that prescribing probiotics in preterm babies is a difficult task, because we face important data heterogenicity and many aspects are not clearly specified, such as multiple- or single-strain administration, duration, dose or which of the preterms are more suitable for best benefits. In the light of these data, the ESPGHAN Committee on Nutrition and the ESPGHAN Working Group for Probiotics and Prebiotics investigated through network meta-analysis the strains with the highest efficacy for preventing major neonatal morbidities in preterm infants(4). After that, they were able to make and publish a guide for the possible use of probiotics in preterm infants, declaring all the limitations and possible future research directions(5).
Reasons for using probiotics in preterms
The background of using probiotics to treat or prevent any disease is based upon the ability to modulate gut microbiota, in both children and adults. Health benefits are enabled through a multitude of actions summarized as follows (Figure 1): colonization of dysbiotic intestinal communities and competitivity with pathogenic strains, production of volatile short chain fatty acids, cell mediated actions – adhesion, receptor antagonism, mucin production, immune system modulation and influence on gut-brain axis(6).
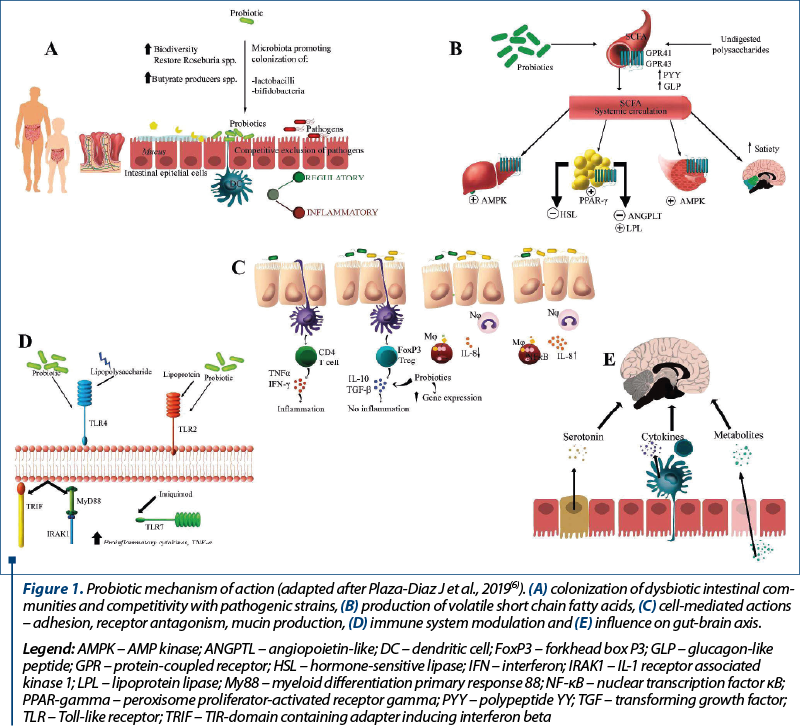
Gut colonization begins at birth in healthy full-term infants and continues as a dynamic process along with toddlers’ developmental stages(7). Being born preterm alters the process of bacterial colonization as a consequence of a multifactorial etiology, such as immaturity of the gut environment, exposure to antibiotics, and supplemental feeding with formula(8).
Modulating preterm newborn microbiota is challenging because there is hard to characterize their gut constellations, which is not univocal, as it can be influenced by factors like birth delivery method, place of birth, first feeding practices, indication of antibiotic usage in the first days of live, and NICU microbial environment itself(9). In the meantime, there is a continuous research in terms of causality between dysbiosis and preterm main comorbidities, and the available data are sometimes confused about probiotic-induced changes in gut microbiota and surveillance eventually(10). In other words, we know the gut problem of these newborns and its capacity of influencing short- and long-term consequences, we begin to identify their microbiome particularities, and we are trying to modulate the intestinal microbial populations through feeding practices and probiotic usage. Of course, wide medical evidence data base is the gold standard of new as valid recommendations, but the process of meta-analysis is not an easy task due to the enormous studies heterogenicity, making them probably underpowered, in terms of sample size calculation, single pre-specified outcomes (i.e., mortality, NEC, LOS, feeding tolerance), single or multiple probiotic strains implicated, duration of treatment, timing of probiotic administration, which group of infants would likely benefit from the intervention and, last but not least, the type of implicated species. Despite all these limitations, the actual ESPGHAN guideline about probiotics in preterm babies is a first step in aiding clinical practice.
Single or combination probiotics
in preterm infants
For a long period, using multiple strains probiotics was considered superior in actions than single strains supplementation. The ongoing ESPGHAN guideline is not very specific regarding this recommendation, but argues in favour of using only those probiotics, either single or in combination, that have validated safety characteristics(5). Most of the recent published data suggest that using a combination of different strains may have advantages over single probiotic organisms(11-13). One of the most important researches in this area was the network meta-analysis performed by van den Akker et al., who carried out a comparison of evidence across several competing interventions(4). The analysis include single- or multi-strain studies reporting on NEC, LOS or overall mortality. The results suggested that only a minority of interventions, regardless of being single-strain or multi-strain species, was found to improve the established outcomes and there was no clear overlap of certain strains which were significantly effective on multiple outcome domains. The final remarks concluded that understudied species might reflect some negative results, as well as the extreme variety of probiotics used in neonatal period. One of the most important weaknesses of this kind of study is the variability of the strains and the protocols theirselves. Considering the complexity of the normal gut microbiome and the various pathogenic pathways involved in NEC, probiotics have to address a multitude of vulnerable points in order to be effective. As a consequence, different probiotic strains may exert their improvement in clinical by different pathways, and in generally the most used probiotics have some nonspecific benefits and share a generic protection(14). This is the reason why a multi-strain or multi-species probiotic is more likely to be more effective than a single-strain product, because it can address several pathways. Additive and/or synergistic effects of those kinds of combinations and mixtures enable a broader efficacy spectrum because they can offer a greater diversity of the microbiota. In the meantime, some other studies argue that a combination of probiotic strains does not mandatory ensure their efficacy, taking into account the fact that sometimes there are mutual inhibitory properties due to differential gene expression(15).
Dose and species considerations
The most important probiotic strains used and tested in the randomized controlled trials (RCTs) included in the actual guideline which fulfilled the safety criteria and were investigated in no less than 247 infants (per group) were: B. breve BBG-001 (YIT4010), L. reuteri DSM 17938, L. rhamnosus GG ATCC 53103, S. boulardii CNCM I-745, the combination of B. bifidum NCDO 1453 with L. acidophilus NCDO 1748 (ATCC 4356, LA37, or NCIMB 30316), and the combination of B. infantis Bb-02, B. lactis Bb-12, and Str. thermophilus TH-4.
The position paper recommends the use of L. rhamnosus GG ATCC 53103 at a dose ranging from 1 x 109 CFU to 6 x 109 CFU in order to reduce NEC stage 2 ore 3, with a low grade of evidence, using the combination of B. infantis Bb-02, B. lactis Bb-12, and Str. thermophilus TH-4 at a dose of 3 to 3.5×108 CFU (of each strain) because it might reduce NEC stage 2 or 3. In the meantime, there is a negative recommendation regarding the use of L. reuteri DSM 17938 in preterm infants to reduce the risk of mortality, NEC stage 2 or 3, or sepsis; the panel states that L. reuteri DSM 17938 is a partially D-lactate producing strain for which there is insufficient safety data available in preterm infants(5). Other several single or combination of strains were analyzed in the same publication and the conclusion regarding the use of the combination of B. bifidum NCDO 1453 (currently reclassified as B. longum) with L. acidophilus NCDO 1748 (ATCC 4356, LA37, or NCIMB 30316) in preterm infants in order to reduce the risk of mortality, NEC stage 2 or 3, or sepsis was that no recommendation can be made in either direction. On the other hand, there is a recommendation against using B. breve BBG-001 in terms of reducing the risk of mortality, NEC stage 2 or 3, or sepsis. S. boulardii involves safety concerns, thus the panel does not recommend the routine use of this probiotic, due to the risk of fungaemia, mainly in patients using a central venous catheter.
Optimal start or treatment duration
There is a huge variability regarding the timing of the first probiotic administration and the duration of treatment as well(4). In order to reach the main health benefits of probiotic treatment, mainly NEC prevention, this should be initiated before and during the most vulnerable period, which is not immediately after birth. Studies included in the meta-analysis used probiotics starting the first days of life, as well as after 2 weeks and had a total treatment duration varying from 2 weeks up to 4-6 weeks. The recommendation regarding this issue is based upon clinical experience adapted locally, according to the patient’s ongoing risk of NEC.
Breastfeeding versus formula feeding
in preterm infants
An issue of controverse is whether probiotics are required in a breastfed infant, considering that breast milk is an ideal complex of nutrients, naturally provided, that contains a lot of bioactive elements such as lactoferrin, probiotics and human milk oligosaccharides. Most studies support improving the clinical outcomes while using probiotics in breastmilk-fed preterm infants with greater benefits than in formula-fed infants.
Conclusions
Although using probiotics during the neonatal period is sustained by researchers with enthusiasm, the preterm infant presents a particular gut environment, and some interactions might not have the proposed benefits. Anyway, the actual recommendations advise for using some specific strains, and recommend against the use of other. Further randomized controlled trials exploring probiotic supplementation in clinical outcomes of preterm are still needed.
Bibliografie
-
Harrison MS, Goldenberg RL. Global burden of prematurity. Semin Fetal Neonatal Med. 2016;21:74–79.
-
Platt MJ. Outcomes in preterm infants. Public Health. 2014;128:399–403.
-
Dermyshi E, Wang Y, Yan C, Hong W, Qiu G, Gong X, Zhang T. The “Golden Age” of probiotics: A systematic review and meta-analysis of randomized and observational studies in preterm infants. Neonatology. 2017;112:9–23.
-
van den Akker CHP, van Goudoever JB, Szajewska H, et al. Probiotics for Preterm Infants: A Strain-Specific Systematic Review and Network Meta-analysis.
-
J Pediatr Gastroenterol Nutr. 2018;67(1):103-22.
-
van den Akker CHP, et al. Probiotics and Preterm Infants: A Position Paper by the European Society for Paediatric Gastroenterology Hepatology and Nutrition Committee on Nutrition and the European Society for Paediatric Gastroenterology Hepatology and Nutrition Working Group for Probiotics and Prebiotics. Journal of Pediatric Gastroenterology and Nutrition. 2020;70(5):664-680.
-
Plaza-Diaz J, Ruiz-Ojeda FJ, Gil-Campos M, Gil A. Mechanisms of Action of Probiotics. Adv Nutr. 2020;11(4):1054.
-
Perez-Muñoz ME, Arrieta MC, Ramer-Tait AE, Walter J. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome. 2017;5:48.
-
Groer MW, Luciano AA, Dishaw LJ, Ashmeade TL, Miller E, Gilbert JA. Development of the preterm infant gut microbiome: a research priority. Microbiome. 2014;2:38.
-
Iozzo P, Sanguinetti E. Early dietary patterns and microbiota development: Still a way to go from descriptive interactions to health-relevant solutions. Front Nutr. 2018;5:5.
-
Sanders ME, Guarner F, Guerrant R, Holt PR, Quigley EMM, Sartor RB, Sherman PM, Mayer E. An update on the use and investigation of probiotics in health and disease. Gut. 2013;62:787–796.
-
Chang HY, Chen JH, Chang JH, Lin HC, Lin CY, Peng CC. Multiple strains probiotics appear to be the most effective probiotics in the prevention of necrotizing enterocolitis and mortality: An updated meta-analysis. PLoS ONE. 2017;12:e0171579.
-
Thomas JP, Raine T, Reddy S, Belteki G. Probiotics for the prevention of necrotising enterocolitis in very low-birth-weight infants: A meta-analysis and systematic review. Acta Paediatr. 2017;106;1729–1741.
-
Embleton ND, Zalewski S, Berrington JE. Probiotics for prevention of necrotizing enterocolitis and sepsis in preterm infants. Curr Opin Infect Dis. 2016;29:256–261.
-
Patole S. Probiotics for preterm infants. The story searching for an end. Indian Pediatr. 2017;54:361–362.
-
Ouwehand AC, Invernici MM, Furlaneto FAC, Messora MR. Effectiveness of multistrain versus single-strain probiotics current status and recommendations for the future. J Clin Gastroenterol. 2018;52(1):S35-S40.
Articole din ediţiile anterioare
Necesarul energetic şi proteic al sugarilor născuţi prematur – recomandări de nutriţie postexternare
Incidenţa restricţiei de creştere extrauterină în momentul externării din maternitate rămâne mare, în ciuda introducerii alimentaţiei parenterale...