A number of feline blood borne agents have been grown, amplified or have induced serum antibodies in the serum of cats with clinical signs like fever. The purpose of this comprehensive review is to provide an update on the diagnosis and management of fever associated with Anaplasma phagocytophilum, Bartonella spp, Ehrlichia spp., haemoplasmas, and Rickettsia spp. infections of cats. Please also see the AAFP Panel report on feline bartonellosis (www.catvets.com) and the ACVIM Ehrlichia Consensus Statement (www.acvim.org) for further information.
Anaplasmosis. Cats have shown to be susceptible to A. phagocytophilum infection after experimental inoculation. DNA of A. phagocytophilum has been amplified from naturally exposed cats in several countries including Spain, Portugal, Sweden, Denmark, Ireland, and the United States. Basically, any country or region that has Lyme disease in dogs will have A. phagocytophilum infection in cats. As in dogs, A. phagocytophilum is transmitted by Ixodes ticks and so infections of cats are likely to be most common in these areas.
While the pathogenesis of disease associated with A. phagocytophilum in cats is unknown, some cats experimentally inoculated with A. phagocytophilum developed anti-nuclear antibodies and increased IFN-gamma mRNA suggesting that an immune pathogenesis of disease may contribute to the clinical findings. Fever, anorexia, and lethargy are the most common clinical abnormalities in naturally infected cats. However, in a recent experimental study in my laboratory, cats infected with A. phagocytophilum by exposure to wild caught Ixodes spp. ticks remained clinically normal over the 70-day study period in spite of being PCR positive for A. phagocytophilum DNA in blood for several weeks (In press, 2015). Whether or not this agent is associated with chronic recurrent fever in cats is unknown. Cats with fever in endemic areas can have blood smears examined cytologically but morulae are less commonly detected than in dogs. Some commercial laboratories offer serologic testing or PCR assays to amplify A. phagocytophilum DNA from blood. Approximately 30% of cats with proven clinical infections induced by A. phagocytophilum are seronegative when first assessed serologically, but most of the proven cases evaluated to date have ultimately seroconverted. Some mountain lions with A. phagocytophilum DNA amplified from blood have been serum antibody negative and so a single negative antibody result in an acutely infected cat does not exclude infection. Therefore, cats with suspected anaplasmosis may need convalescent serum samples to prove infection. Alternately, antibody testing could be combined with PCR testing of whole blood in acute cases. One of the canine assays for detection of A. phagocytophilum antibodies (SNAP4DXPlus, IDEXX Laboratories) can be used to detect antibodies in cat serum.
The following is an abstract from ACVIM that will soon be published.
Detection of feline antibodies against a novel Anaplasma phagocytophilum peptide (AP-4) after exposure to wild-caught adult ixodes scapularis
(MR Lappin1, R. Chandrashekar2, B. Stillman2, J Liu2.
1. Department of Clinical Sciences, Colorado State University, Ft. Collins, CO.
2. IDEXX Laboratories, Portland ME.)
Cats that are exposed to Anaplasma phagocytophilum (AP) infected Ixodes scapularis ticks develop serum antibody responses to an immunodominant peptide (P44) used in a commercially available assay that is licensed for use with canine serum. The purpose of this study was to evaluate feline antibody responses to a novel AP peptide (AP-4) as a method for evaluating for early evidence of AP infection in cats.
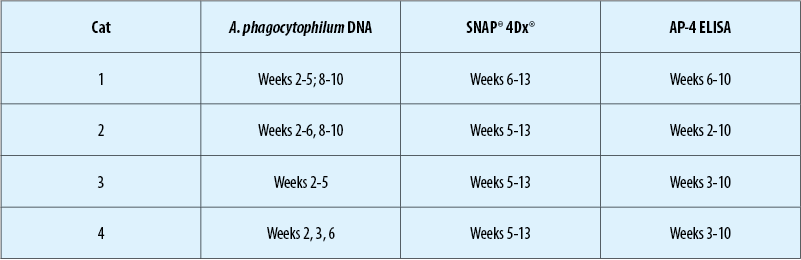
Young, adult, mixed sex research cats (n = 4) were used in this pilot study with IACUC approval. Each of the cats was shown to be negative for antibodies against AP using a commercially available kit (SNAP® 4Dx®, IDEXX Laboratories) and negative for AP DNA in blood by use of a commercially available conventional PCR assay (Veterinary Diagnostic Laboratory, Colorado State University). Using the pre-inoculation samples, an ELISA for detection of antibodies against the AP-4 was optimized. Cats were infected with AP by exposure to wild-caught Ixodes scapularis. Blood for AP PCR assay and for serum separation for assessment for AP antibodies by SNAP® 4Dx® and AP-4 ELISA were collected prior to tick attachment and then on weeks 1-10 after tick attachment.
Each cat had AP DNA amplified from blood and each cat developed detectable AP antibodies in serum by both assays. Antibodies against AP were detected prior to those detected by the commercially available kit for 3 of 4 cats.
The results suggest that antibodies against the AP-4 peptide may be useful for the early detection of AP exposure in cats and that further studies from samples collected from naturally exposed cats should be performed.
Several antibiotics have been administered to naturally infected cats, but all cats in 2 studies became clinically normal within 24 to 48 hours after initiation of tetracycline or doxycycline administration and recurrence was not reported(1,3). While clinically normal, two cats were still PCR positive 17 days and 90 days after treatment (of 21 to 30 days duration) which suggests that treatment with tetracyclines for 21 to 30 days may be inadequate for eliminating the organism from the body. In a recent experimental study, bacteremia as determined by PCR assay was limited over several weeks (Lappin et al., unpublished data, 2012).
Bartonellosis. A number of Bartonella spp. including B. henselae, B. clarridgeiae, B. koehlerae, B. quintana and B. bovis have been cultured or amplified from client-owned cats with fever. Fever following experimental inoculation with B. henselae has been documented in a number of studies including a recent study in our laboratory where the CSU-1 strain of B. henselae induced significant fever in three of six cats after exposure to infected C. felis. None of the six cats administered imidacloprid-moxidectin in that study became infected or febrile. However, not all strains or Bartonella spp. induce fever in all cats; for example in the imidacloprid-moxidectin study, cats inoculated with the same strain intravenously failed to develop fever. Whether fever will occur during Bartonella spp. infection is likely a complex interaction that is influenced by both host and organism factors.
As B. henselae, B. clarridgeiae, B. koehlerae are transmitted by fleas, bacteremia and antibody positive rates can be very high. For example, serum antibodies were detected in 93% of cats housed in a North Carolina shelter and Bartonella spp. DNA was amplified from the blood of >50% of cats housed in an Alabama shelter. The majority of these cats were thought to be normal which emphasizes that fever from bartonellosis cannot be documented by test results alone. In one study of pair matched cats with or without fever, serum Bartonella antibodies detected by ELISA or Western blot immunoassay were not correlated to the presence of fever. In addition, serum antibody test results are negative in between 3 and 15% of bacteremic cats. Thus, if a cat with fever is to be evaluated for Bartonella spp. infection the combination of blood culture or PCR assay on blood, and serologic testing will detect the greatest number of cats that are currently or previously infected. Febrile cats that are seronegative and negative for Bartonella spp. in blood by culture or Bartonella spp. DNA in blood are unlikely to have the organism as the cause of fever.
If fever from bartonellosis is suspected in a cat, administration of doxycycline or a fluoroquinolone is generally effective. The AAFP Panel Report recommended doxycycline at 10 mg/kg, PO, daily for 7 days as the initial therapeutic trial. If a positive response is achieved, continue treatment for 2 weeks past clinical resolution of disease or for a minimum of 28 days. If a poor response is achieved by day 7 or doxycycline is not tolerated and bartonellosis is still considered a valid differential diagnosis, fluoroquinolones are appropriate second choices. In experimental or field studies, administration of enrofloxacin or orbifloxacin have led to rapid resolution of fever in cats with presumed bartonellosis. Azithromycin is now considered contraindicated because of rapid induction of resistance.
Ehrlichiosis. Ehrlichia‑like bodies or morulae have been detected in peripheral lymphocytes or monocytes of naturally exposed cats in a number of countries including the United States, Kenya, France, Brazil, and Thailand. One study of cats in North America amplified DNA consistent with E. canis from naturally infected cats. However, other studies of cats in endemic areas (Florida and Arizona) have failed to amplify Ehrlichia spp. DNA from the blood of cats. In 2 separate experimental studies, we have failed to amplify monocytotropic Ehrlichia spp. from blood or detect seroconversion in cats inoculated SQ with different strains of cultured E. canis (Lappin and Breitschwerdt, unpublished observations, 2007; Lappin and Little, unpublished observations, 2010). These results indicate the E. canis-like DNA amplified from naturally-infected cats may be from a different Ehrlichia spp. more infective to cats, not all E. canis stains will infect cats, not all cats are susceptible to infection by E. canis, or SQ inoculation is not an effective method for infecting cats with E. canis. Some cats with suspected clinical ehrlichiosis have seroreacted to E. canis or N. risticii morulae.
Fever is one of the reported clinical abnormalities detected in cats with suspected ehrlichiosis and so testing may be indicated in these cats. However, a validated serological assay is not currently available and some cats with E. canis-like DNA in blood were seronegative. In contrast, most A. phagocytophilum infected cats have strongly positive antibody test results. Positive serologic test results occur in both healthy and clinically ill cats, and so a diagnosis of clinical ehrlichiosis should not be based on serologic test results alone. Ehrlichia spp. PCR and gene sequencing can be used to confirm infection and should be considered the tests of choice at this time.
Clinical improvement after therapy with tetracycline, doxycycline, or imidocarb dipropionate was reported for most cats with suspected mononcytopic ehrlichiosis. However, for some cats a positive response to therapy was a criterion for the diagnosis of ehrlichiosis. The current recommendation of the ACVIM Infectious Disease Study Group is to give doxycycline (10 mg/kg PO q24h for 28 days). For cats with treatment failure or those intolerant of doxycycline, imidocarb diproprionate can be administered (5 mg/kg IM or SQ twice, 14 days apart). Salivation and pain at the injection site are the common adverse effects and imidocarb efficacy is in question for the treatment of canine monocytotropic ehrlichiosis.
Hemoplasmosis. Fever has been detected in some cats with infection by Mycoplasma haemofelis, “Candidatus Mycoplasma haemominutum”, or “Candidatus M. turicensis”. In multiple studies of experimentally infected cats, M. haemofelis is apparently the most pathogenic species. Dual infection with hemoplasmas may potentiate pathogenesis of disease. In one study, cats with chronic “Candidatus Mycoplasma haemominutum” infection had more severe anemia and longer duration of anemia when experimentally infected with M. haemofelis when compared to cats infected with M. haemofelis alone. In one abstract, we reported an association between M. haemofelis and fever in cats without anemia. Clinical signs of disease depend on the degree of anemia, the stage of infection, and the immune status of infected cats. Direct transmission may occur with the hemoplasmas and so the agents should be on the differential list for cats with a history of fighting.
Diagnosis of hemoplasmosis is based on demonstration of the organism on the surface of erythrocytes on examination of a thin blood film or by PCR assay results. Organism numbers fluctuate and so blood film examination can be falsely negative up to 50% of the time. The organism may be difficult to find cytologically, particularly in the chronic phase. Thus, PCR assays are the tests of choice due to sensitivity.
Doxycycline is often administered as a flavored suspension (to avoid esophageal strictures) at 10 mg/kg, PO, every 24 hours for a minimum of 7-10 days. In cats intolerant of doxycycline, enrofloxacin given at 5 mg/kg, PO, every 24 hours for 14 days was tolerated and is equally effective or more effective than doxycycline. Administration of marbofloxacin or orbifloxacin gives similar results. Azithromycin was not effective for the treatment of hemoplasmosis in one study. Most drug protocols have failed to eliminate infection and so at this time there is no clinical utility to repeat PCR testing. The owners should be warned that recurrences may occur but are unusual.
Feline rickettsiosis. Rickettsia spp. are obligate intracellular gram negative bacteria that are divided into the spotted fever group and the typhus group. Cats can be infected by Rickettsia felis and have been shown to have antibodies against R. rickettsii. Rickettsia felis DNA has been amplified from C. felis, C. canis, and Pulex irritans; these fleas have a worldwide distribution. Ctenocephalides felis is a biological vector for R. felis; the organism can be transmitted transovarially and transtadially within the flea. Rickettsial infection is suspected to be a cause of fever in cats but this has not been well documented (Bayliss). While we have commonly amplified R. felis from C. felis (67.4% of flea extracts in one study), we have not amplified the organism from the blood of healthy cats or cats with fever. However, in one study of cats with fever we showed R. felis and R. rickettsii antibody prevalence rates in cats in the USA to be 5.6% and 6.6%, respectively, but neither organism was amplified from blood. These results prove that cats are sometimes exposed to spotted fever group organisms but further data are needed to determine significance of diseases associations. Because clinical illness in cats has not been documented, optimal treatment is unknown. However, based on results in dogs, doxycycline or a fluoroquinolone would be logical choices.
Summary. There are other infectious agents of cats that are either blood borne or associated with bacteremia that should be on the differential list for cats with appropriate clinical findings and geographical locale including feline leukemia virus, feline immunodeficiency virus, Cytauxzoon felis, Hepatozoon spp., Francisella tularensis, and Yersinia pestis.