Clozapine-resistant forms of schizophrenia are not exceptionally met in clinical practice, and epidemiological studies confirm this observation. Between one-third and two-thirds of patients undergoing treatment with clozapine present the so-called “ultra-resistant” form of schizophrenia – i.e., the persistence of significant psychotic symptoms even though the previously mentioned antipsychotic was administered for a sufficient duration, in adequate doses, and with good therapeutic adherence. This explains why it is necessary to identify therapeutic strategies for these patients, especially if the significant negative functional impairment of clozapine-resistant schizophrenia is taken into account. For this purpose, a narrative analysis of the literature was carried out, dedicated to the evaluation of pharmacological, psychotherapeutic and neuromodulatory methods that can be recommended in these patients as clozapine augmentation strategies. Alternatives to clozapine for clozapine-resistant patients or cases of individuals who cannot tolerate this antipsychotic are also explored in the current review. First- and second-generation antipsychotics added to clozapine, switching from clozapine to another antipsychotic or combination of antipsychotics, using high doses of antipsychotics in resistant cases, combining clozapine with mood stabilizers, antidepressants, anxiolytics or other pharmacological agents are analyzed from their efficacy and tolerability properties. Based on the analyzed reports, adding atypical antipsychotics with a complementary pharmacodynamic profile to clozapine for positive symptoms, or mood stabilizers or antidepressants for affective or negative symptoms in patients with ultra-resistant schizophrenia could be useful, for short durations and under adequate monitoring.
Therapeutic options in ultra-resistant schizophrenia. Pharmacological interventions (I)
Opţiuni terapeutice în schizofrenia ultrarezistentă. Intervenţii farmacologice (I)
First published: 27 aprilie 2023
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/Psih.72.1.2023.7932
Abstract
Rezumat
Formele de schizofrenie rezistente la clozapină nu sunt rar întâlnite în practica clinică, fapt, de altfel, confirmat şi de studiile epidemiologice. Între o treime şi două treimi dintre pacienţii care urmează tratament cu clozapină prezintă aşa-numita formă „ultrarezistentă” de schizofrenie, caracterizată prin persistenţa unor simptome psihotice semnificative, deşi antipsihoticul menţionat a fost administrat pentru o durată suficientă, în doze adecvate şi cu o aderenţă terapeutică bună. Prin urmare, se impune identificarea unor strategii de management terapeutic în cazul acestor pacienţi, în special dacă se are în vedere şi puternicul impact funcţional negativ al schizofreniei rezistente la clozapină. În acest scop, a fost realizată o analiză narativă a literaturii, dedicată evaluării metodelor farmacologice, psihoterapeutice şi neuromodulatorii care pot fi recomandate ca strategii de augmentare a clozapinei. De asemenea, sunt explorate în această analiză şi alternativele la clozapină în cazul pacienţilor rezistenţi la clozapină sau la cei care nu pot tolera acest antipsihotic. Antipsihoticele de prima şi a doua generaţie adăugate la clozapină, trecerea de la clozapină la un alt antipsihotic sau o combinaţie de antipsihotice, utilizarea unor doze mari de antipsihotice în cazurile rezistente, combinarea clozapinei cu timostabilizatoare, antidepresive, anxiolitice ori cu alţi agenţi farmacologici sunt investigate din perspectiva eficacităţii şi a tolerabilităţii. Pe baza dovezilor analizate, adăugarea la clozapină a unor antipsihotice atipice cu profil farmacodinamic complementar pentru simptomele pozitive ori a timostabilizatoarelor sau antidepresivelor pentru simptome afective sau negative la pacienţii cu schizofrenie ultrarezistentă poate fi realizată cu precauţie, pe durate scurte şi sub o monitorizare adecvată.
Introduction
The concept of “ultra-resistant schizophrenia” (URS), or “clozapine-resistant schizophrenia” (CRS), has multiple definitions, and still, there is no agreement about clearly defined pharmacological and clinical diagnostic criteria for this entity(1). In order to correctly diagnose CRS, several preconditions are needed: (a) the diagnosis of schizophrenia should be verified in case of clozapine nonresponse; (b) the absence of significant comorbid conditions should be checked, a process which includes screening for organic and toxic pathologies, or other psychiatric disorders (e.g., mood disorders, personality disorders); and (c) social and psychological stressors that could negatively interfere with the treatment effects should be addressed(2). Lack of therapeutic adherence is important to monitor, as an essential predictor of nonresponsivity to clozapine. The plasma levels of clozapine are to be determined on a regular basis in case of nonresponse, and a minimum value of 350-600 ng/ml is recommended(2,3). A trial of clozapine of three to six months at an efficient therapeutic plasma concentration is recommended prior to augmenting or changing this antipsychotic(2).
Which are the next steps when clozapine fails? Multiple augmentation strategies for the clozapine regimen, including pharmacological, psychotherapeutic and neuromodulatory interventions, have been suggested for CRS, but their level of evidence still needs further exploration before becoming clinical recommendations. Therefore, a narrative review of the available therapeutic options for this specific population is considered useful for clinicians who have to manage difficult cases of clozapine-resistant or clozapine-intolerant schizophrenia. For the purpose of this review, all schizophrenia spectrum disorders have been included in the evaluation, because no significant neuropathological differences were detected between them. Also, no large-scale, comparative analysis detected specific treatment responsivity to certain antipsychotics within these subpopulations.
It is important to make the distinction between nonresponse and partial response to clozapine, due to the fact that the preferred therapeutic management approaches may be quite different – i.e., switch on a different intervention or the add-on of a distinct therapy, respectively. Also, where clozapine intolerance has been used as an inclusion criterion in a clinical trial or as a descriptor of a case report, this aspect was emphasized due to its clinical importance for the therapeutic decision.
Pathogenesis of ultra-resistant schizophrenia
Although the concept of CRS has quite a long history, a very small number of research has been dedicated to the pathogenesis of this disorder. The CRS phenotype (defined here by lack of remission in patients who received clozapine and at least two conventional or atypical antipsychotics) has been explored from the hypothesis of genetic contributors to its onset(4). Significant associations between Disrupted in Schizophrenia 1 (DISC1) gene missense variants and this pathology have been supported by evidence(4). On the other hand, in a case-control study (106 Caucasian patients diagnosed with schizophrenia, clozapine-resistant schizophrenia included, versus 127 healthy controls), no differences in the distribution of 15 genetic variants in uncoupling protein (UCP) 2, UCP4 and UCP5 were detected between the two groups(5). Still, one haplotype including UCP4 was less frequently identified in CRS individuals(5).
Chronic peripheral inflammation has been a phenomenon explored in relation to therapeutic resistance in schizophrenia(6). In a study that recruited 609 stabilized schizophrenia patients (out of which 60 were considered CRS cases, defined by ongoing treatment with clozapine and a Positive and Negative Syndrome Scale [PANSS] score of at least 70), high sensitivity (hs) CRP was evaluated as a marker for peripheral inflammation(6). The CRS status was independently associated with a high level of hs-CRP, illness duration, and a lower level of functioning(6). These observations raise the question of the possible utility of anti-inflammatory treatments in clozapine-resistant schizophrenia as add-ons to clozapine.
A systematic review (n=25 studies) explored the correlation between neuroimagistic findings, the CRS clinical status, and the effects of clozapine(7). The five studies that reported on differences between treatment-resistant (including URS cases) and non-treatment-resistant patients offered no replicated conclusion(7). Hypometabolism in the prefrontal cortex, but also hypermetabolism in the basal ganglia, and structural changes in the corpus callosum might contribute to the onset of treatment resistance, based on comparisons between these patients and healthy controls(7). Clozapine may increase the hypoactivation of the prefrontal cortex in patients with treatment resistance, but without a clinically significant impact(7). Clozapine administration also might correlate with decreased metabolism in the thalamus and basal ganglia, which has been associated with the efficacy of this atypical antipsychotic(7).
Exploring other non-dopaminergic systems in schizophrenia – and especially in clozapine-resistant schizophrenia – may be important for detecting new ways to enhance the efficacy of clozapine. Especially the glutamatergic system has been studied in the pathogenesis of schizophrenia, with N-methyl-D-aspartate (NMDA) receptors dysfunction on gamma-amino-butyric acid (GABA) interneurons causing disinhibition of glutamate neurons projecting to the basal ganglia(8). Serotonergic and endocannabinoid systems may also play a role in the onset of schizophrenia, but their contribution to the CRS onset has been less explored(9,10).
The pharmacogenomics of clozapine response should also be taken into consideration when treatment resistance is analyzed. Most of the available data regarding the modulation of the efficacy and tolerability of clozapine by genetic factors have been obtained by using the candidate gene approach(11). Variants of the CYP isoenzymes (CYP1A2 and 2C19), serotonin and dopamine receptors genes have been explored in relation to clozapine efficacy, and limited evidence supports that single nucleotide polymorphisms of HTR2A and HT3A genes may correlate with significant response to this antipsychotic(11,12). Also, positive associations were observed between DRD1, DRD2, DRD3, DRD4, catechol-O-methyltransferase (COMT) and solute carrier family 6 member 3 (SLC6A3) genes and clozapine response(11,13).
Predictive factors for CRS have been reported, with younger age of onset, lower premorbid functioning, the prolonged first episode of psychosis, higher rate of relapse, and higher dose of antipsychotics used in the first two years after the diagnosis being the most relevant(14,15). Clozapine-resistant schizophrenia has been correlated with more severe premorbid social dysfunctions in late adolescence and with a longer period until clozapine initiation when compared to non-URS patients(15).
Further research in the field of pathogenic factors involved in the onset of CRS would be very useful for multiple clinical reasons, starting from preventative-focused interventions, to specific, personalized treatments in patients with certain genetic vulnerabilities.
Objective and methodology
A narrative review dedicated to finding evidence-based interventions for clozapine-resistant schizophrenia has been conducted by searching the main electronic databases (PubMed, Cochrane, Clarivate/Web of Science, EMBASE) but also the main repositories of clinical trials (www.clinicaltrials.gov, www.clinicaltrialsregister.eu and www.who.int/clinical-trials-registry-platform). Also, references within the main reviewed papers were searched for supplementary information, when this was considered necessary. The search paradigm was “clozapine resistance” OR “ultra-resistant” AND “schizophrenia” OR “schizophrenia spectrum disorders” OR “schizoaffective disorder” AND “antipsychotics” OR “mood stabilizers” OR “antidepressants” OR “anxiolytics” OR “add-on medication” OR “neuromodulation” OR “psychotherapy”. All papers were reviewed without limits regarding the time or language they were published. Both primary and secondary reports were included in the review.
In the first part of this paper, pharmacological interventions will be analyzed, while in the second part, all other types of therapies and therapeutic perspectives will be reviewed.
Results
The search on the electronic databases and clinical trials repositories found 51 sources reporting on antipsychotics administered in clozapine-resistant schizophrenia, out of which 21 were case reports or case series, 25 were clinical trials or retrospective studies, four systematic reviews or meta-analyses, and one therapeutic guideline. Mood stabilizers in CRS were explored in six reports, consisting of one case presentation, three clinical trials/retrospective studies, and two systematic reviews/meta-analyses. References about the efficacy and/or tolerability of antidepressants in clozapine-resistant schizophrenia have been found in five reports – one case study and four clinical trials/retrospective studies. Data about the use of anxiolytics in CRS were sparse, with only one review being found about this topic. Other pharmacologic agents were explored in five sources, out of which four were clinical trials/retrospective studies and one review/meta-analysis. For each agent, the papers will be presented starting with case reports/case series, followed by clinical trials, systematic reviews/meta-analyses and, where possible, therapeutic guidelines will end the data presentation.
Antipsychotics added to clozapine
Almost one-third of CRS patients receive augmentation with an antipsychotic in clinical practice(16). According to a Cochrane review dedicated to the efficacy and tolerability of combining clozapine and different antipsychotics (n=5 studies; N=309 participants), based on a low to very low quality of evidence, it could not be concluded the superiority of a certain combination over another(17). The same review reported several significant differences between clozapine combinations for global ratings of mental status therapeutic response and change, discontinuation rate, and weight changes(17). Therefore, the clinician should be aware that there are certain nuances of the clinical effect of antipsychotics in CRS patients, even though no antipsychotic could be considered superior to others.
The most explored combinations of antipsychotics in patients with schizophrenia are those between clozapine and atypical antipsychotics, either second- or third-generation agents (Figure 1).
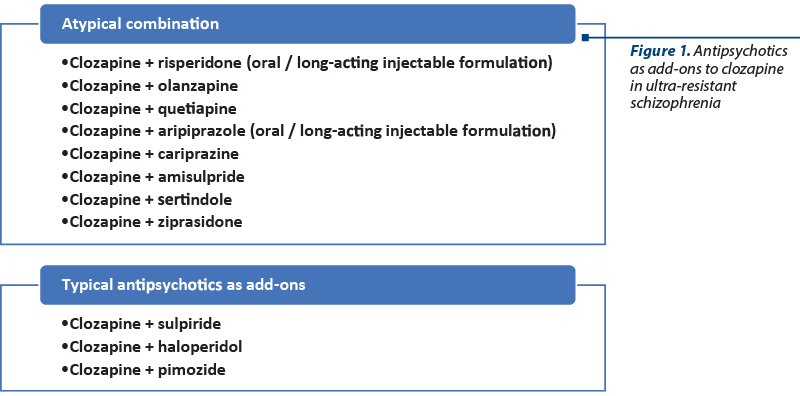
A combination of risperidone and clozapine was initiated in two patients with schizophrenia who did not previously respond to either antipsychotic administered alone(18). In the first case, a 500 mg/day dosage of clozapine was initially administered but withdrawn due to partial efficacy and lack of adherence to the monitoring of adverse events, and risperidone 6 mg was introduced but later discontinued due to worsening of the patient’s status(18).
A case series of four patients investigated the effects of combined treatment with risperidone microspheres and clozapine in cases where nonadherence to oral treatment was detected(19). After the initiation of the long-acting injectable antipsychotic, the duration of hospitalization and the rate of readmission to the hospital declined(19). This favorable effect was observed in the long term in three patients who continued this combined treatment for up to 3.5 years, and their social skills were also markedly improved(19).
A trial of risperidone (up to 4.5 mg/day) and clozapine (up to 300 mg/day) was initiated, with favorable evolution and no adverse events for the following two years of monitoring(18). The other patient also responded well to the addition of clozapine (up to 400 mg/day) to the risperidone treatment (up to 6 mg/day) during a period of six weeks of active monitoring(18).
In a randomized, controlled trial, patients with schizophrenia and partial or lack of response to clozapine monotherapy (N=40) received either a placebo or risperidone (up to 6 mg/day) for 12 weeks(20). The Brief Psychiatric Rating Scale (BPRS) recorded significant improvements in both groups at weeks 6 and 12, but the evolution of patients treated with clozapine + risperidone was significantly more favorable(20). The Scale for the Assessment of Negative Symptoms (SANS) also reflected significant improvements in patients who received active treatment, and the adverse events profile was similar between groups(20). No additional increments of weight gain, agranulocytosis, or seizures were detected in the combined treatment group versus the clozapine plus placebo group(20). Therefore, risperidone may be useful as an add-on to clozapine for the improvement of the negative symptoms in patients with schizophrenia.
In another randomized controlled trial, patients with schizophrenia and partial response to clozapine (N=30) received risperidone (≤6 mg/day) or placebo as an add-on for six weeks(21). A significantly superior improvement of the main efficacy outcome (PANSS-positive subscale score) was observed in patients with combined treatment(21). The tolerability profile was similar between the two groups, without significant differences regarding extrapyramidal symptoms, weight gain and QTc interval; no difference in the clozapine levels was detected between groups, either(21). Sedation and higher levels of plasma prolactin levels were, however, more frequently reported in patients who received clozapine plus risperidone(21).
A review (n=15 studies; N=86 patients with schizophrenia or schizoaffective disorder; mean dose of clozapine = 474.2 mg/day) assessed the effects of risperidone (mean dose 4.6 mg/day) as an add-on for a mean duration of 7.9 weeks in case of partial responsivity(22). The psychopathology was significantly improved in 43% of the enrolled patients, with a lower dosage of risperidone and a longer duration of the trial being associated with a better outcome(22). Extrapyramidal symptoms, sedation and hypersalivation were the main adverse events reported(22).
Clozapine and olanzapine combination has been explored in case reports, with mostly positive results. In a 48-year-old male diagnosed with a schizoaffective disorder, the augmentation of clozapine (600 mg/day; plasma level 888 ng/ml) with lithium (1800 mg/day; plasma level 0.5 mEq/l) was not effective, while the addition of risperidone and haloperidol was also associated with a minimal impact on psychotic symptoms(23). The clozapine was up-titrated to 900 mg/day, but without success, therefore a combination of clozapine and olanzapine was recommended, and the patient accepted(23). The positive symptoms improved, and the clozapine dose could be decreased to 600 mg/day, while olanzapine was up-titrated to 15 mg/day(23). Sialorrhea was noted as a side effect in this patient(23). In a 29-year-old female with chronic schizophrenia, clozapine could not be tolerated in a dose of 650 mg/day (sedation, sialorrhea, instability), therefore a reduction to 250 mg/day was necessary (plasma level 102 ng/ml); olanzapine was initiated at 10 mg/day and raised to 15 mg/day(23). The BPRS score decreased and her social functionality improved(23). In yet another case report, a 55-year-old woman with schizoaffective disorder was stabilized on clozapine 300 mg/day, but she presented significant sedation, which severely impaired her functioning(24). After a failed attempt to switch to olanzapine (up to 25 mg/day), the combination of 10 mg/day olanzapine and 100 mg/day clozapine led to favorable results in four days, with symptoms severity decrease and functionality improvements(24). The favorable evolution persisted up to 198 months of follow-up(24).
Although these three case reports did not include any significant adverse events, the association between olanzapine and clozapine should be very carefully monitored. A case of lethal acute colonic pseudo-obstruction in a patient treated with this combination of antipsychotics has been reported, with both agents being associated with gastrointestinal hypomotility as a potential adverse event(25).
Clozapine and quetiapine were explored as an association in an open-label, non-randomized, 10-month retrospective study which enrolled 65 patients with schizophrenia(26). All patients were initially on 200-800 mg/day of clozapine, and quetiapine was added gradually(26). This strategy allowed for a reduction of the clozapine daily dose, and the quetiapine’s final dose range was 200-800 mg/day(26). This study primarily monitored the metabolic parameters and reported marked total weight loss and improvement in glucose metabolism(26). Drowsiness was the main adverse event reported, but no discontinuation due to this issue was recorded(26).
In another retrospective study (N=1000 charts of patients diagnosed with schizophrenia or schizoaffective disorder treated with clozapine who developed different adverse events and received quetiapine as an add-on), the impact of this combination was favorable(27). A clozapine dosage reduction was possible and no significant adverse events due to the combination of these two antipsychotics were reported(27). Quetiapine was helpful in controlling weight gain, diabetes mellitus, hypersalivation and constipation related to clozapine’s administration(27).
In two in-patients with schizophrenia and suboptimal response to clozapine (675 and 850 mg/day) and low tolerance to their current regimen (somnolence, sialorrhea and weight gain), 15 mg aripiprazole were added(28). Positive symptoms decreased in the next six weeks and the clozapine dose was reduced to 425 mg and 600 mg, respectively(28). The tolerability improved in both patients(28). There is a high synergy between clozapine and aripiprazole, due to their pharmacodynamic profiles, with a good safety profile(28).
The addition of aripiprazole long-acting injectable formula (up to 400 mg/month) to clozapine (the dose of 300 mg/day was reduced to 150 mg/day due to sedation and myoclonus) in a patient with schizophrenia and persistent psychotic symptoms led to significant improvement (>50% severity scores on PANSS, BPRS and Clinical Global Impression – CGI) that persisted up to one year of follow-up(29). The tolerability of this combination was good(29).
A retrospective chart review (N=24 patients treated with clozapine and aripiprazole combination, who presented persistent positive and negative symptoms), concluded a significant improvement in hallucinations and delusions during this treatment regimen(30). Most of the patients were much improved (more than 80% of the cases), but very much improved (>12%) and minimally improved (>4%) patients were also identified, based on the CGI-Improvement (CGI-I) scores after a mean period of 33.8 weeks(30). Their social functioning was also better at the end of the monitoring period, with more than 80% of the patients acquiring new hobbies and activities(30). This combination of drugs was well tolerated and safe, and no discontinuation due to lack of efficacy was reported(30).
A systematic review and meta-analysis of randomized controlled trials with short duration (8-24 weeks) evaluated the effects of the clozapine plus aripiprazole in patients with schizophrenia and concluded the superiority of the combined intervention on the general psychotic symptoms, positive and negative manifestations(31). Surprisingly, no benefit on three cardiometabolic parameters was found – i.e., fasting plasma glucose, triglyceride and HDL cholesterol, but body weight and LDL cholesterol decreased during this treatment compared to clozapine plus placebo(31).
The administration of cariprazine in two cases of treatment-resistant schizophrenia and partial response to clozapine was reported(32). These patients had a long history of schizophrenia (≥10 years) and received previously oral and long-acting injectable antipsychotics, prior to the switch to clozapine(32). The decrease in PANSS scores was observed on all subscales, but mostly on the negative symptoms, which is explainable by cariprazine’s mechanism of action(32). Also, a favorable effect was reported on weight and BMI (both of each decreased during the monitoring period), and the overall tolerability of the combined antipsychotics was good(32).
A case series (N=15 patients with schizophrenia/schizoaffective disorder who were resistant to their ongoing atypical antipsychotic – clozapine, olanzapine, risperidone or ziprasidone) received amisulpride augmentation (mean dose 693.3±279.6 mg/day)(33). There was reported an improvement in these patients’ mental state, except for three patients who did not change compared to baseline(33). This retrospective report included five patients resistant to clozapine (mean dose of 490±174.6 mg/day), previously treated with at least two typical agents or at least one atypical antipsychotic(33). However, the data presented in this study are derived only from clinical observation, with no validated instrument for the measurement of psychosis severity being used.
A case series including 15 patients with schizophrenia initiated on amisulpride augmentation of clozapine concluded that significant (N=6 participants) or marked (N=8) improvement of treatment-resistant positive or negative symptoms could be achieved with this combination(34). The mean doses used were 375 mg/day of clozapine and 527 mg/day of amisulpride(34). Also, a reduction of the clozapine daily dose by 24% versus monotherapy has been possible and led to a reduction of adverse events(34).
In a case report, persistent positive symptoms (mainly auditory hallucinations) and working and social functioning decline were observed during clozapine administration (450 mg/day)(35). The addition of haloperidol (up to 6 mg/day) for three months did not improve this patient’s status and the tolerability worsened (extrapyramidal symptoms, sexual adverse events)(35). A switch from haloperidol to amisulpride (up to 1000 mg/day) was recommended while preserving the clozapine regimen(35). Clinical and functional improvements were reported soon after this switch and the treatment tolerability also increased(35).
Clozapine regimen augmentation using amisulpride or placebo was investigated during 12 weeks in 68 patients with schizophrenia, and the combined treatment led to higher rates of response at the end of the study(36). Negative symptoms were more significantly improved in patients receiving amisulpride(36). Adverse events were more frequently reported in the clozapine plus amisulpride group, but the cost-efficacy analysis indicated the superiority for the combined intervention(36).
A randomized trial enrolled 80 patients randomized on amisulpride plus clozapine or clozapine plus placebo, monitored for 12 weeks(37). At the end-point, the patients who received both antipsychotics had lower PANSS total scores, positive subscale scores and general psychopathology scores(37). The cognitive status also improved in patients who received amisulpride (Repeatable Battery for the Assessment of Neuropsychological Status; RBANS) versus placebo, while the response rate and the clinical global status improvement scores were higher in the active versus placebo group(37).
Sertindole was used as an augmenting agent to the clozapine regimen in a double-blind, placebo-controlled trial, based on its low affinity for cholinergic receptors(38). The patients (N=50; presenting residual symptoms after more than six months) were randomized to sertindole 16 mg or placebo for 12 weeks and monitored for efficacy and safety(38). No superiority was observed for sertindole versus placebo as an augmentation strategy to clozapine at the end-point (according to the PANSS, Global Assessment of Functioning Scale-GAF and CGI scores), although the tolerability of the combined treatment was good(38).
The addition of ziprasidone to clozapine was reported in a 28-year-old woman diagnosed with schizophrenia and resistance to either of the two antipsychotics administered as monotherapy (120 mg and 150 mg, respectively)(39). Their combined administration (120 mg ziprasidone and 75 mg clozapine) improved both positive and negative symptoms (according to PANSS scores) and decreased the adverse events(39). Body weight decreased, while blood pressure, pulse and electrocardiogram remained within the normal range(39). Another case report by the same author referred to a 35-year-old male with schizophrenia, resistant to olanzapine, risperidone, quetiapine and clozapine who received ziprasidone as an add-on agent(40). The clozapine dose was reduced after the ziprasidone initiation, and the positive, negative and affective symptoms improved (based on PANSS scores)(40).
A case series (N=2 outpatients with schizophrenia and suboptimal response to doses of 600-750 mg/day clozapine) reported persistent positive symptoms (auditory hallucinations and persecutory delusions) and adverse events to clozapine (somnolence, sialorrhea and weight gain)(41). Ziprasidone was added (up to 80 mg/day) and the positive symptoms decreased at week 12 (based on BPRS scores), allowing for a reduction of the clozapine dose(41). The tolerability of clozapine increased as soon as its doses were reduced(41).
An open-label trial enrolled nine patients with treatment-resistant schizophrenia who have initiated a clozapine plus ziprasidone regimen and were monitored for six months(42). The mean BPRS score improved in seven patients, and this improvement was significant at the end-point(42). No increase in side effects was reported and an 18% reduction of the clozapine dose was allowed after ziprasidone was added(42).
Also, older reports exist about the combination of clozapine and first-generation antipsychotics. The association of clozapine and sulpiride has been explored in patients with schizophrenia who responded only partially to clozapine. In a case report, a patient who was initiated on clozapine responded well in the domain of positive symptoms (at 700 mg/day), but his status worsened due to the necessity of decreasing the daily dose to 600 mg/day (hypersalivation, drop attacks)(43). Therefore, the clozapine dose was reduced further to 500 mg/day and sulpiride was initiated as an augmenting agent(43). After this change, the psychotic symptoms were remitted and no significant adverse events emerged(43).
In a double-blind, randomized trial, the patients (N=28) treated with sulpiride (600 mg/day) and clozapine improved significantly regarding the positive and negative psychotic symptoms, with a regimen for 10 weeks, compared to those receiving clozapine plus placebo(44). Younger patients and lower scores on the Scale for the Assessment of Positive Symptoms (SAPS) were associated with more favorable evolution during the combined treatment(44).
Other potential benefits of adding sulpiride to clozapine have been suggested in the literature. The association of small doses of sulpiride (150-300 mg/day) to clozapine (100-800 mg/day) might be useful for patients with schizophrenia who present with clozapine-induced hypersalivation(45). In 18 patients monitored for 21 days, the addition of sulpiride decreased the mean Nocturnal Hypersalivation Rating Scale (NHRS) scores and clinically reported sialorrhea, with only three participants presenting minimal sialorrhea at the end-point(45).
A Cochrane review (n=4 studies; N=221 participants with treatment-resistant or prominent negative symptoms of schizophrenia) evaluated the effects of the clozapine plus sulpiride combination versus clozapine ± placebo and concluded that no differences could be detected in the domain of the global state, relapse rate, or clinical response between the two interventions(46). Regarding the adverse events, clozapine plus sulpiride led to more movement disorders and to an increase in plasma prolactin levels, but to diminished appetite loss and abdominal distension in this population(46).
Haloperidol (4 mg/day) was added to clozapine (mean daily dose 450 mg/day) in a double-blind, randomized, controlled trial, in ten patients with schizophrenia and persistent psychotic symptoms(47). The PANSS total scores were improved more in the clozapine plus placebo group at week 10, although the D2 receptors occupancy (in the striatum and cerebellum) increased significantly more in the clozapine + haloperidol group(47). Therefore, no correlation could be established between D2 occupancy and the clinical effect of the haloperidol plus clozapine combination, but the sample size in this study was very small(47).
Pimozide (mean daily dose 6.48 mg/day) was added to clozapine (≥378 ng/ml plasma concentration at baseline) in a double-blind, placebo-controlled label trial that enrolled 53 patients with schizophrenia or schizoaffective disorder, partially responsive or completely nonresponsive to clozapine(48). There was observed no superiority of pimozide over placebo as an augmentation strategy for clozapine, according to the PANSS scores after 12 weeks(48). These results contradict the conclusions of an earlier, open-label trial, conducted by the same team in seven patients with schizophrenia or schizoaffective disorder and residual mild-to-moderate psychotic manifestations (BPRS score at baseline was 51); in this trial, 2-8 mg pimozide were added for a mean period of 32 days to 325-600 mg/day clozapine, and the efficacy outcome (BPRS score) improved significantly (the mean final score was 27)(49).
Comparative trials with antipsychotics as add-ons to clozapine
The reports previously mentioned investigated the efficacy and safety of clozapine plus typical or atypical antipsychotics either without a comparator or versus clozapine monotherapy or clozapine plus placebo. However, more complex research has been conducted, exploring the comparative efficacy and safety of different add-ons to clozapine.
The comparison of clozapine plus amisulpride versus clozapine plus quetiapine in patients (N=50) with schizophrenia who respond only partially to clozapine was realized in an 8-week single-blind randomized study(50). Both groups improved their efficacy outcomes (SANS, SAPS and CGI), but amisulpride was associated with a more favorable evolution than quetiapine(50). The difference between groups was detected at week 3 for CGI scores and at week 6 for the BPRS, SANS and SAPS scores(50). The overall tolerability was good in both groups, determined by Kliniske Undersogelser (UKU) and Simpson Angus Scale (SAS) (50).
Another 12-month, randomized, naturalistic trial explored the comparative efficacy and safety of clozapine plus aripiprazole versus clozapine plus haloperidol in 106 patients with resistant schizophrenia(51). The main outcome was the proportion of patients who discontinued treatment, and there was no significant difference between groups at the end-point(51). The BPRS score change also did not differ in patients depending on the treatment they received and the tolerability was superior for aripiprazole(51).
The associations of clozapine plus ziprasidone and clozapine plus risperidone were compared in an open-label randomized trial that enrolled patients monitored during an acute phase of six weeks, with follow-up visits at 26 and 52 weeks(51). All the included patients (N=24) presented treatment-resistant psychotic symptoms during clozapine monotherapy (≥300 mg/day; plasma levels ≥200 µg/L)(52). Improvements in positive and negative symptoms (assessed by PANSS and SANS) and a decrease in depressive manifestations (HAMD) were reported in both groups(52). The patients who were treated with ziprasidone plus clozapine reported a mild increase in akathisia, but the tolerability of clozapine improved(52). The favorable effects of the combined treatment, clozapine plus ziprasidone/risperidone, persisted for up to 52 weeks(52). The reduction of co-administered medication was also possible after the introduction of ziprasidone/risperidone(52).
Antipsychotic-based strategies after the discontinuation of clozapine
Clinicians tend to avoid switching from clozapine to another antipsychotic, due to the risk of psychosis exacerbation. However, empirical studies to support this belief are very limited(53). Several reports about other strategies that could be applied when clozapine is not tolerated or there is no response to it have been identified in the literature (Figure 2). However, switching back to atypical antipsychotics in case of clozapine nonresponse may be effective in some patients(54).
In patients who discontinued clozapine for various reasons (N=321 cases), the initiation of a second-generation antipsychotic was associated with a better evolution than the starting of a first-generation agent(55). Reinitiation of clozapine and switching from clozapine to risperidone or olanzapine were the most efficient strategies(55). In the case of reinitiation of clozapine, it should be noticed that previous serious adverse events to this agent preclude its readministration(55).
Another real-world comparative effectiveness of antipsychotics initiated after clozapine was discontinued, based on an analysis of data from the Finnish registry, included 2250 patients with schizophrenia(56). Reinitiation of clozapine, oral olanzapine and antipsychotic polypharmacy were strategies associated with the lowest readmission rates(56). Treatment failure was the least probable in patients who received aripiprazole long-acting injectable formulation, clozapine reinitiation and oral olanzapine(56). The analysis of mortality risk indicated reinitiation of clozapine and oral olanzapine as the safest interventions in these patients(56).
A case report presents a favorable response to amisulpride, which gradually increased to 1200 mg/day, in a 34-year-old patient with nonresponsive negative symptoms and functional impairment, to 500 mg/day clozapine, administered for four years(57). The switch from clozapine to amisulpride was gradual. The pharmacogenomic analysis found that this patient was a poor CYP3A5 metabolized and CYP2D6 intermediate metabolizer, therefore amisulpride was chosen because of its minimal hepatic metabolism(57). The therapeutic response to amisulpride was favorable, with a significant PANSS score reduction during 12 weeks(57).
The patients who could not respond or tolerate clozapine were switched to olanzapine (5-25 mg/day) in an open-label study (N=48)(58). The total BPRS and PANSS scores decreased from the baseline by 14% and 20%, respectively, after 18 weeks(58). The response was achieved by 30% of these patients, based on PANSS scores change, and by 55.6% based on BPRS scores(58). Both positive and negative scores changed during the olanzapine treatment(58). The overall tolerability was good, and no clinically relevant dyscrasias were detected, not even in patients who developed these adverse events during clozapine administration(58). Another open-label trial (N=19 patients discontinued from clozapine) reported a response rate of 42% after switching to olanzapine(59). However, another five patients decompensated and required hospitalization(59). In yet another four cases, clozapine was reinitiated due to the worsening of the clinical status(59). The BPRS total score decreased significantly at the end-point, and the responders required a shorter duration of clozapine treatment prior to the switch and a lower dose of clozapine(59).
A retrospective study (N=33 patients with treatment-resistant schizophrenia who received clozapine) evaluated the effects of switching from clozapine to paliperidone palmitate administered monthly, followed by every three months (PP1M and PP3M, respectively)(60). Switching from clozapine to PP3M was associated with lower concomitant antipsychotic and benzodiazepine administration, but with more biperiden use(60). The BMI, glucose, cholesterol, and triglycerides values also decreased after switching from clozapine to PP3M(60).
Zotepine has a pharmacodynamic profile similar to that of clozapine, and in a 12-week prospective, randomized, rater-blind study, 59 patients with schizophrenia resistant to clozapine were randomized to continuing their current medication or switching to zotepine(61). The mean daily doses were 397.1 mg for clozapine and 377.1 mg for zotepine(61). The BPRS scores increased during the switch to zotepine and the adverse effects (extrapyramidal symptoms, hyperprolactinemia) were more frequent in the same patients(61). Therefore, switching from clozapine to zotepine needs careful monitoring.
Another case series reported on three patients with schizophrenia who had persistent positive and negative symptoms despite the clozapine administration(53). Cariprazine was titrated while clozapine decreased gradually, and a significant improvement was reported up to 14 months of monitoring(53). The favorable effect of cariprazine was noted on positive and negative manifestations of psychosis in all cases(53).
In two patients with chronic schizophrenia who could not respond to or tolerate clozapine, loxapine was initiated(62). Adherence to the loxapine treatment was superior to that of the clozapine regimen because of the lack of sialorrhea or periodic blood sample collection(62). When used in low doses (less than 50 mg/day), loxapine has similar tolerability to that of second-generation antipsychotics(62). Loxapine was efficient in another patient, a 57-year-old male with severe paranoid schizophrenia, resistant to clozapine (699 ng/ml plasma level), who was cross-titrated to loxapine(63). The patient was finally discharged, on a regimen including 100 mg of loxapine twice daily, combined with lithium and valproate ER(63).

The combination of two antipsychotics, other than clozapine, has been suggested as a possible intervention in ultra-resistant cases of schizophrenia(54). Patients unresponsive to the first antipsychotic may improve after the addition of a second antipsychotic, and the benefits of the first agent may be preserved after the initiation of the second agent(54). There are also disadvantages – i.e., unnecessarily high doses, the addition of adverse events, negative pharmacologic interactions, risk of increased mortality, higher costs, low level of evidence and low treatment adherence(54).
A retrospective chart review (N=7 patients with acute exacerbation of schizophrenia) concluded that the combination of amisulpride (mean dose 485.7 mg) and olanzapine (mean dose 21.4 mg) led to improvements in refractory symptoms, according to the GAF and CGI scores(64). This combination allowed for a lower dose of each antipsychotic – i.e., the reduction of olanzapine by 21% and of amisulpride by 26%(64). The adverse events (e.g., body weight gain and extrapyramidal symptoms) decreased in several cases secondary to the dose reduction(64).
In a case report, a 46-year-old patient with schizophrenia who received treatment with clozapine developed rebound insomnia after the antipsychotic’s discontinuation and switch to sertindole(65). Z-drugs and chloral hydrate did not succeed in significantly controlling insomnia in the long term, therefore the addition of 300 mg quetiapine was initiated(65). The subjective quality of sleep improved after the quetiapine add-on, with a good sleep latency and a lower number of awakenings during the night(65). No adverse events were reported in this case(65).
A patient with treatment-resistant schizophrenia and intolerance to clozapine was diagnosed with a comorbid prolactin-secreting microadenoma(66). A combination of olanzapine and quetiapine was recommended in this case which led to an almost complete resolution of psychotic symptoms, without raising prolactin levels(66).
Higher doses of atypical antipsychotics were considered as a possible therapeutic option in clozapine-resistant patients who need to be switched to a different agent. The efficacy of this strategy has, however, not been validated by good-quality clinical evidence. The use of a higher dose of olanzapine for hospitalized patients has been described in the literature – for example, the average dose increased from 17.4 mg to 22.5 mg/day between 1997 and 2003, without a valid reason(67). There are case reports describing the benefits of using 60 mg/day olanzapine, and a good benefit-risk ratio, while double-blind trials dedicated to this topic (although few) concluded there might be helpful to increase the dose over 20 mg/day in selected, treatment-resistant patients(67). Weight gain and hyperprolactinemia could be reported in patients with high doses of olanzapine, therefore caution is needed in this case(67).
According to the British Association of Psychopharmacology Guidelines (2020), the use of a higher dose of antipsychotics or a combination of antipsychotics is not recommended for clozapine-resistant schizophrenia, except when all other options have been exhausted(68). The use of this regimen should be carefully monitored, with adverse events, efficacy and adherence as the main outcomes(68). A combination of antipsychotics or a high dose of antipsychotics should be considered for a limited duration, and only on case-by-case analysis exceptions can be made(68).
Mood stabilizers as add-ons to clozapine
A meta-analysis of 22 randomized controlled trials evaluated the effects of adding four antiepileptics to clozapine in ultra-resistant schizophrenia cases(69). Topiramate, lamotrigine and sodium valproate were superior to clozapine monotherapy when the total psychopathology was considered, with topiramate and sodium valproate also being efficient for positive and general symptoms(69). Valproate was well tolerated, while topiramate add-on was associated with a high all-cause discontinuation rate(69).
A meta-analysis (n=5 trials; N=161 patients with schizophrenia receiving clozapine) investigated the effects of lamotrigine as an add-on for ultra-resistant cases(70). The results were in favor of lamotrigine in both primary and secondary outcome measures, with an NNT (number needed to treat) value of 4, suggesting that 20-30% of clozapine-resistant patients may benefit from this strategy(70). Rash was the most frequently reported adverse event in these patients(70).
The addition of lithium in 20 patients with schizophrenia or schizoaffective disorder partially responsive to clozapine was explored in a randomized controlled trial(71). Patients with schizoaffective disorder improved with lithium on CGI, cognitive measures and PANSS scores, while patients with schizophrenia did not change significantly in any of the outcomes(71). Safety measures did not fluctuate, but two patients developed reversible neurotoxic reactions(71). Favorable effects were observed on hematologic parameters (total leucocyte number and absolute granulocyte counts) in patients with clozapine plus lithium versus clozapine plus placebo(71).
Topiramate was proven useful as an augmenting agent to clozapine in a 12-week open-label study that enrolled 20 patients with schizophrenia with suboptimal response to clozapine(72). According to the BPRS scores change, topiramate led to an improvement of clinical status (-14% reported to baseline), a decrease of body weight (-2.5% to baseline), and it was well tolerated (except for paresthesia which was the most common adverse event)(72). However, these results were only partially confirmed by a double-blind, placebo-controlled trial, which enrolled 43 patients with incomplete responses to clozapine and monitored them for 24 weeks(73). Topiramate (200 mg/day) led to a mild improvement in clinical symptomatology and a trend toward cognitive complaints was observed in the active treatment versus placebo group(73).
Divalproex was added to clozapine in treatment-resistant patients with schizophrenia and compared to clozapine plus lithium and clozapine monotherapy in a retrospective study (N=24 participants)(74). The total BPRS scores at six months were improved in all treatment groups, but the response was faster (at week 4) in patients with clozapine plus divalproex/lithium versus monotherapy with clozapine(74). Adverse events occurred similarly in all groups, without significant differences between them (sedation, tachycardia, gastrointestinal symptoms, confusion or dizziness)(74). Lithium was associated with a higher weight gain and with higher blood glucose levels(74).
In a patient diagnosed with a long history of schizophrenia, who presented repeated aggressive behavior, nonresponsive to typical (e.g., haloperidol) and atypical (e.g., risperidone, olanzapine) antipsychotics, the combined therapy including clozapine had to be discontinued due to severe hematologic adverse events(75). Also, in this patient, electroconvulsive therapy (ECT) did not lead to the remission of psychotic symptoms(75). When gabapentin (up to 2700 mg/day) was added to haloperidol (30 mg/day), a positive effect was observed within four weeks in the domain of persecutory delusions and aggressive manifestations(75). Gabapentin was again added when delusional symptoms reappeared, and it again succeeded in controlling these manifestations(75). The modulatory actions of gabapentin on the GABA-ergic and glutamatergic neurotransmission might explain its favorable clinical impact when co-administered with antipsychotics for the control of psychosis in treatment-resistant cases(75). The tolerability of gabapentin in this case report was not systematically assessed.
Antidepressants as add-ons in CRS
The concomitant administration of selective serotonin reuptake inhibitors (SSRIs) and clozapine has been associated with potentially significant pharmacokinetic interactions(76). Fluvoxamine is a potent CYP1A2 inhibitor and it can increase clozapine blood levels, and reports about similar increases exist in relation to sertraline co-administration(76). Therefore, several authors recommend caution when SSRIs and clozapine are co-administered, and regular clozapine plasma levels should be determined(76).
Fluvoxamine (50 mg/day) was added to clozapine after a steady state was reached during clozapine monotherapy in 16 patients with schizophrenia(77). The serum concentrations of clozapine and its metabolites were significantly increased (2-5 times) after fluvoxamine was added, but there was no increase in the adverse events rate(77). Also, the psychopathology improved during the combined fluvoxamine plus clozapine treatment(77).
Clozapine and paroxetine were combined in a 32-year-old patient with schizophrenia (persistent delusions and negative symptoms) and obsessive-compulsive features which severely affected his daily functioning(78). A dose of 30 mg/day of paroxetine was added to 200 mg/day of clozapine (plasma levels 150 ng/ml) and this led to a decrease in the Yale-Brown Obsessive Compulsive Scale (Y-BOCS) score during the 14 weeks of monitoring(78). Also, his positive symptoms decreased, according to the BPRS scores, by 40%(78).
Fluoxetine was also explored as an augmenting agent to the clozapine regimen in a double-blind, parallel-group comparison study that enrolled 33 patients monitored for eight weeks(79). These patients presented persistent positive or negative symptoms despite adequate treatment with clozapine(79). The results of this trial did not support the use of fluoxetine used as an add-on to clozapine, because the results in patients receiving drugs combination were similar to those from patients receiving clozapine + placebo(79).
An 8-week double-blind, randomized, controlled trial explored the effects of 30 mg adjunctive mirtazapine in clozapine in patients (N=24) with schizophrenia(80). The favorable effects of this combination were detected on the SANS total scores, especially on “apathy/avolition” and “anhedonia/asociality”(80). Also, the final BPRS total score was lower in patients who received mirtazapine versus those who were on placebo(80).
Duloxetine was used as an adjunctive to clozapine in patients with schizophrenia (N=33) and persistent symptoms of psychosis in a 16-week double-blind, randomized, controlled trial focused on the efficacy and safety of this combination(81). Duloxetine (60 mg/day) was superior to the placebo for the improvement of negative and general psychopathology, but did not distinguish itself from the placebo regarding the effect on cognitive functioning(81).
Anxiolytics as add-ons to clozapine
The use of benzodiazepines as augmenting agents in clozapine-resistant patients with schizophrenia is based on very limited data(82). However, anxiety and negative symptoms may benefit from the addition of benzodiazepines to clozapine(82). Chlordiazepoxide and diazepam, long-acting benzodiazepines, seem to be an option for these patients, in case of specific symptoms and according to a low degree of evidence(82). Safety aspects should be considered when benzodiazepines and clozapine are used concomitantly, because of the risk of cardiovascular or respiratory accidents, disinhibition and dysphoria(82,83).
Other drugs administered as add-ons to clozapine
Besides mood stabilizers, antidepressants and anxiolytics or different pharmacological agents have been explored as add-ons to clozapine (Figure 3). The glutamatergic theory of schizophrenia suggests that the enhancement of the NMDA receptor function may reduce the psychotic symptoms(84). The potentiation of the glycine site of NMDA receptor has been explored as a therapeutic mechanism, with full agonists like glycine and D-serine, and glycine transporter-1 (GlyT-1) inhibitor, sarcosine, added to antipsychotics in patients with schizophrenia(84). These drugs did not show any significant benefit when added to clozapine, which indicates once again the unicity of this antipsychotic(84).

Memantine was added to clozapine in a randomized, double-blind, placebo-controlled study, starting from the same NMDA receptor hypofunction hypothesis(85). Memantine or placebo was added to 52 patients with treatment-resistant schizophrenia, and the results at week 26 showed the superiority of memantine on a composite memory score and on the PANSS negative subscale score(85). The overall tolerability of clozapine plus memantine was good(85). Verbal and visual memory improved significantly during this combined treatment(85).
Minocycline was explored as an adjuvant to clozapine in patients with schizophrenia or schizoaffective disorder (N=52) with persistent positive symptoms in a 10-week double-blind, placebo-controlled trial(86). The effect of minocycline on global cognitive and positive symptoms was not statistically superior to placebo, but significant improvements were seen in working memory, avolition, and anxiety/depression symptoms(86).
Donepezil augmentation of clozapine monotherapy was explored in an 18-week double-blind cross-over study with eight patients diagnosed with schizophrenia and residual symptoms of psychosis(87). There was observed no difference between groups in the total positive and negative symptom scale scores of PANSS, but three patients improved significantly according to their total PANSS scores on donepezil versus only one on placebo(87). No significant difference between groups was observed on Calgary Depression Scale, Simpson Angus Scale or CGI-S(87).
Ginkgo biloba extract (120 mg/day) was compared to the placebo as an add-on to clozapine in treatment-resistant schizophrenia in a randomized controlled study with 42 patients monitored for 12 weeks(88). The Ginkgo biloba extract was associated with favorable results on negative symptoms (SANS scores), but had no effect on positive or general symptomatology(88).
Conflict of interest: none declared
Financial support: none declared
This work is permanently accessible online free of charge and published under the CC-BY.

Bibliografie
-
Campana M, Falkai P, Siskind A, Wagner E. Characteristics and definitions of ultra-treatment-resistant schizophrenia - A systematic review and meta-analysis. Schizophr Res. 2021;228:218-226. https://doi.org/10.1016/j.schres.2020.12.002.
-
Roerig JL. Clozapine augmentation strategies. Ment Health Clin. 2019;9(6):336-348. doi: 10.9740/mhc.2019.11.336.
-
Wagner E, Kane JM, Correll CU, Howes O, Siskind D, Honer WG, et al. Clozapine combination and augmentation strategies in patients with schizophrenia - recommendations from an international expert survey among the treatment response and resistance in psychosis (TRIPP) working group. Schizophr Bull. 2020;46(6):1459-1470. https://doi.org/10.1093/schbul/sbaa060.
-
Mouaffak F, Kebir O, Chayet M, Torjman S, Vacheron MN, Millet B, et al. Association of Disrupted in Schizophrenia 1 (DISC1) missense variants with ultra-resistant schizophrenia. Pharmacogenomics. 2011;11(4):267-73. doi: 10.1038/tpj.2010.40.
-
Mouaffak F, Kebir O, Bellon A, Gorebitch R, Tordjman S, Viala A, et al. Association of a UCP4 (SLC25A27) haplotype with ultra-resistant schizophrenia. Pharmacogenomics. 2011;12(2):185-93. doi: 10.2217/pgs.10.179.
-
Fond G, Godin O, Boyer L, Berna F, Andrianarisoa M, Coulon N, et al. Chronic low-grade peripheral inflammation is associated with ultra resistant schizophrenia. Results from the FACE-SZ cohort. Eur Arch Psychiatry Clin Neurosci. 2019;269(8):985-992. doi: 10.1007/s00406-018-0908-0.
-
Nakajima S, Takeuchi H, Plitman E, Fervaha G, Gerretsen P, Caravaggio F, et al. Neuroimaging findings in treatment-resistant schizophrenia: A systematic review: Lack of neuroimaging correlates of treatment-resistant schizophrenia. Schizophr Res. 2015;164(1-3):164-75. doi: 10.1016/j.schres.2015.01.043.
-
Sîrbu CA, Manole AM, Vasile M, Toma GS, Dobrican LR, Vîrvara DG, Vasiliu O. Cannabinoids - a new therapeutic strategy in neurology. RJMM. 2022;3(CXXV):349-355. doi: 10.55453/rjmm.2022.125.3.1
-
Potkin SG, Kane JM, Correll CU, Lindenmayer JP, Agid O, Marder SR, et al. The neurobiology of treatment-resistant schizophrenia: paths to antipsychotic resistance and a roadmap for future research. NPJ Schizophr. 2020;6(1):1. doi:10.1038/s41537-019-0090-z.
-
Selvaraj S, Arnone D, Cappai A, Howes O. Alterations in the serotonin system in schizophrenia: a systematic review and meta-analysis of post-mortem and molecular imaging studies. Neurosci Biobehav Rev. 2014;45:233-45. doi: 10.1016/j.neubiorev.2014.06.005.
-
Numata S, Umehara H, Ohmori T, Hashimoto R. Clozapine pharmacogenetic studies in schizophrenia: Efficacy and agranulocytosis. Front Pharmacol. 2018;9:1049. doi: 10.3389/fphar.2018.01049.
-
Gressier F, Porcelli S, Calati R, Serretti A. Pharmacogenetics of clozapine response and induced weight gain: a comprehensive review and meta-analysis. Eur Neuropsychopharmacol. 2016;26(2):163-185. doi: 10.1016/j.euroneuro.2015.12.035.
-
Mauro MC, Paletta S, Maffini M, Colasanti A, Dragogna F, Altamura AC. Clinical pharmacology of atypical antipsychotics: an update. EXCLI J. 2014;13:1163-91.
-
Budişteanu M, Andrei E, Lica F, Hulea DS, Velicu AC, Mihăilescu I, et al. Predictive factors in early onset schizophrenia. Exp Ther Med. 2020;20(6):210.
-
Chan SKW, Chan HYV, Honer WG, Bastiampillai T, Suen YN, Yeung WS, et al. Predictors of treatment-resistant and clozapine-resistant schizophrenia: A 12-year follow-up study of first-episode schizophrenia-spectrum disorders. Schizophr Bull. 2021;47(2):485-494. doi: 10.1093/schbul/sbaa145.
-
Mouaffak F, Tranulis C, Gourevitch R, Poirier MF, Douki S, Olié JP, et al. Augmentation strategies of clozapine with antipsychotics in the treatment of ultraresistant schizophrenia. Clin Neuropharmacol. 2006;29(1):28-33. doi: 10.1097/00002826-200601000-00009.
-
Barber S, Olotu U, Corsi M, Cipriani A. Clozapine combined with different antipsychotic drugs for treatment-resistant schizophrenia. Cochrane Database Syst Rev. 2017;3(3):CD006324. doi: 10.1002/14651858.CD006324.pub3.
-
Morera AL, Barreiro P, Cano-Muñoz JL. Risperidone and clozapine combination for the treatment of refractory schizophrenia. Acta Psychiatr Scand. 1999;99(4):305-6.
-
Kim SH, Jung DC, Ahn YM, Kim YS. The combined use of risperidone long-acting injection and clozapine in patients with schizophrenia non-adherent to clozapine: a case series. J Psychopharmacol. 2010;24(7):981-6.
-
Josiassen RC, Joseph A, Kohegyi E, Stokes S, Dadvand M, Paing WW, Shaughnessy RA. Clozapine augmented with risperidone in the treatment of schizophrenia: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2005;162(1):130-6. doi: 10.1176/appi.ajp.162.1.130.
-
Yağcioğlu AEA, Akdede BBK, Turgut TI, Tümüklü M, Yazici MK, Alptekin K, et al. A double-blind controlled study of adjunctive treatment with risperidone in schizophrenic patients partially responsive to clozapine: efficacy and safety.
-
J Clin Psychiatry. 2005;66(1):63-72. doi: 10.4088/jcp.v66n0109.
-
Kontaxakis VP, Ferentinos PP, Havaki-Kontaxaki BJ, Paplos KG, Pappa DA, Christodoulou GN. Risperidone augmentation of clozapine: a critical review. Eur Arch Psychiatry Clin Neurosci. 2006;256(6):350-5. doi: 10.1007/s00406-006-0643-9.
-
Gupta S, Sonnenberg SJ, Frank B. Olanzapine augmentation of clozapine. Ann Clin Psychiatry. 1998;10(3):113-5. doi: 10.1023/a:1022350221192.
-
Rhoades E. polypharmacy of 2 atypical antipsychotics. J Clin Psychiatry. 2000;61(9):678-80. doi: 10.4088/jcp.v61n0914e.
-
Bouhouis RH, van Verschuer VMT, Zaal RJ, Dieleman S. Lethal acute colonic pseudo-obstruction in a patient using a combination of olanzapine and clozapine. J Clin Psychopharmacol. 2022;42(5):511-13.
-
Reinstein M, Sirotovskaya L, Jones L, Mohan S, Chasanov MA. Effect of clozapine-quetiapine combination therapy on weight and glycaemic control. Clin Drug Investig. 1999;18:99-104.
-
Reinstein MJ, Sonnenberg JG, Mohan SC, Jones LE. Use of quetiapine to manage patients who experienced adverse effects with clozapine. Clin Drug Investigation. 2003;23:63-67. https://doi.org/10.2165/00044011-200323010-00008.
-
Ziegenbein M, Sieberer M, Calliess IT, Kropp S. Combination of clozapine and aripiprazole: a promising approach in treatment-resistant schizophrenia. Aust N Z J Psychiatry. 2005;39(9):840-1. doi: 10.1111/j.1440-1614.2005.01688_2.x.
-
Sepede G, Di Iorio G, Spano MC, Lorusso M, Sarchione F, Santacroce R, et al. A case of resistant schizophrenia successfully treated with clozapine/long-acting injectable aripiprazole combination. Clin Neuropharmacol. 2016;39(6):322-324. doi: 10.1097/WNF.0000000000000191.
-
Karunakaran K, Tungaraza TE, Harbone GC. Is clozapine-aripiprazole combination a useful regime in the management of treatment-resistant schizophrenia? J Psychopharmacol. 2007;21(4):453-6.
-
Srisurapanont M, Suttajit S, Maneeton N, Maneeton B. Efficacy and safety of aripiprazole augmentation of clozapine in schizophrenia: a systematic review and meta-analysis of randomized-controlled trials. J Psychiatr Res. 2015;62:38-47. doi: 10.1016/j.jpsychires.2015.01.004.
-
De Berardis D, Rapini G, Olivieri L, Giardini A, De Lauretis I, Serroni N, et al. Cariprazine add-on in inadequate clozapine response: A report on two cases. Clin Neuropsychopharmacol Neurosci. 2021;19(1):174-178.
-
Lerner V, Bergman J, Borokhov A, Loewenthal U, Miodownik C. Augmentation with amisulpride for schizophrenic patients nonresponsive to antipsychotic monotherapy. Clin Neuropharmacol. 2005;28(2):66-71.
-
Zink M, Knopf U, Henn FA, Thome J. Combination of clozapine and amisulpride in treatment-resistant schizophrenia - case reports and review of the literature. Pharmacopsychiatry. 2004;37(1):26-31. doi: 10.1055/s-2004-815471.
-
Porcelli S, Serretti A. Clozapine augmentation with amisulpride. J Psychiatry Neurosci. 2014;39(6):E38-39. doi: 10.1503/jpn.140094.
-
Barnes TR, Leeson VC, Paton C, Marston L, Davies L, Whittaker W, et al. Amisulpride augmentation in clozapine-unresponsive schizophrenia (AMICUS): a double-blind, placebo-controlled, randomized trial of clinical effectiveness and cost-effectiveness. Health Technol Assess. 2017;21(49):1-56. doi: 10.3310/hta21490.
-
Zhu MH, Liu ZJ, Hu QY, Yang JY, Jin Y, Zhu N, et al. Amisulpride augmentation strategy therapy improves cognitive performance and psychopathology in clozapine-resistant treatment-refractory schizophrenia: a 12-week randomized, double-blind, placebo-controlled trial. Military Medical Research. 2022;9:59.
-
Nielsen J, Emborg C, Gydesen S, Dybbro J, Aagaard J, Haderup K, et al. Augmenting clozapine with sertindole: a double-blind, randomized, placebo-controlled study. J Clin Psychopharmacol. 2012;32(2):173-8.
-
Zink M, Mase E, Dressing H. Combination of ziprasidone and clozapine in treatment-resistant schizophrenia. Hum Psychopharmacol. 2004;19(4):271-3.
-
Zink M, Mase E, Dressing H. Ziprasidone-augmentation of clozapine. Psychiatr Prax. 2004;31(5):259-61. doi: 10.1055/s-2003-814821.
-
Ziegenbein M, Calliess IT. Clozapine and ziprasidone: a useful combination in patients with treatment-resistant schizophrenia. J Neuropsychiatry Clin Neurosci. 2006;18(2):246-7. doi: 10.1176/jnp.2006.18.2.246.
-
Ziegenbein M, Kropp S, Kuenzel HE. Combination of clozapine and ziprasidone in treatment-resistant schizophrenia: an open clinical study. Clin Neuropharmacol. 2005;28(5):220-4. doi: 10.1097/01.wnf.0000183446.58529.30.
-
Stubbs JH, Haw CM, Staley CJ, Mountjoy CQ. Augmentation with sulpiride for a schizophrenic patient partially responsive to clozapine. Acta Psychiatr Scand. 2000;102(5):390-3. doi: 10.1034/j.1600-0447.2000.102005390.x.
-
Shiloh R, Zemishlany Z, Aizenberg D, Rawan M, Schwartz B, Dorfman-Etrog P, et al. Sulpiride augmentation in people with schizophrenia partially responsive to clozapine. A double-blind, placebo-controlled study. Br J Psychiatry. 1997;171:569-73. doi: 10.1192/bjp.171.6.569.
-
Kreinin A, Ephstein S, Sheinkman A, Tell E. Sulpiride addition for the treatment of clozapine-induced hypersalivation: preliminary study. Isr J Psychiatr Relat Sci. 2005;42(1):61-3.
-
Wang J, Omori IM, Fenton M, Soares B. Sulpiride augmentation for schizophrenia. Cochrane Database Syst Rev. 2010;(1):CD008125. doi: 10.1002/14651858.CD008125.pub2.
-
Mossaheb N, Sacher J, Wiesegger G, Klein N, Spindelegger CJ, Asenbaum S,
-
et al. Haloperidol in combination with clozapine in treatment-refractory patients with schizophrenia. European Neuropsychopharmacology. 2006;16(4):S416.
-
Friedman JI, Lindenmayer JP, Alcantara F, Bowler S, Parak M, White L,
-
et al. Pimozide augmentation of clozapine inpatients with schizophrenia and schizoaffective disorder unresponsive to clozapine monotherapy. Neuropsychopharmacology. 2011;36(6):1289-95. doi: 10.1038/npp.2011.14.
-
Friedman J, Ault K, Powchik P. Pimozide augmentation for the treatment of schizophrenic patients who are partial responders to clozapine. Biol Psychiatry. 1997;42(6):522-3. doi: 10.1016/S0006-3223(97)00227-8.
-
Genç Y, Taner E, Candansayar S. Comparison of clozapine-amisulpride and clozapine-quetiapine combinations for patients with schizophrenia who are partially responsive to clozapine: a single-blind randomized study. Adv Ther. 2007;24(1):1-13. doi: 10.1007/BF02849987.
-
Cipriani A, Accordini S, Nosè M, Purgato M, Girlanda F, Tansella M, Barbui C. Aripiprazole versus haloperidol in combination with clozapine for treatment-resistant schizophrenia: a 12-month, randomized, naturalistic trial. J Clin Psychopharmacol. 2013;33(4):533-7. doi: 10.1097/JCP.0b013e318296884f.
-
Kuwilsky A, Krumm B, Englisch S, Dressing H, Zink M. Long-term efficacy and tolerability of clozapine combined with ziprasidone or risperidone. Pharmacopsychiatry. 2010;43(6):216-20. doi: 10.1055/s-0030-1254089.
-
Duque-Yemail JD, Avila JC. Switching clozapine to cariprazine in three patients with persistent symptoms of schizophrenia: A case series. Neuropsychiatr Dis Treat. 2022;18:1433-1440. doi: 10.2147/NDT.S367922.
-
Pandarakalaman JP. Combination therapy for treatment resistant schizophrenia. BJMP. 2019;12(2):a016.
-
Stam N, Taipale H, Tanskanen A, Isphording L, Okhuijsen-Pfeifer C, Schuiling-Veninga CCM, et al. Persistence of antipsychotic use after clozapine discontinuation: A real-world study across antipsychotics. Clin Transl Sci. 2020;13(6):1170-1177. doi: 10.1111/cts.12801.
-
Luykx JJ, Stam N, Tanskanen A, Tiihonen J, Taipale H. In the aftermath of clozapine discontinuation: comparative effectiveness and safety of antipsychotics in patients with schizophrenia who discontinue clozapine. Br J Psychiatry. 2020;217(3):498-505. doi: 10.1192/bjp.2019.267.
-
Pehlivanidis A, Spyropoulou AC, Tourkantonis A, Papadimitriou GN. Amisulpride monotherapy in a patient with clozapine-resistant schizophrenia. Clin Neuropharmacol. 2010;33(3):168. doi: 10.1097/WNF.0b013e3181dc4e3d .
-
Dossenbach NRK, Beuzen JN, Avnon M, Belmaker RH, Elizur A, Mark M,
-
et al. The effectiveness of olanzapine in treatment-refractory schizophrenia when patients are nonresponsive to or unable to tolerate clozapine. Clin Ther. 2000;22(9):1021-34. doi: 10.1016/s0149-2918(00)80082-x.
-
Henderson DC, Nasrallah RA, Goff DC. Switching from clozapine to olanzapine in treatment-refractory schizophrenia: safety, clinical efficacy, and predictors of response. J Clin Psychiatry. 1998;59(11):585-8. doi: 10.4088/jcp.v59n1105.
-
Martínez-Andrés JA, García-Carmona JA. Switching from clozapine to paliperidone palmitate-3-monthly improved obesity, hyperglycemia and dyslipidemia lowering antipsychotic dose equivalents in a treatment-resistant schizophrenia cohort. Int Clin Psychopharmacol. 2020;35(3):163-169.
-
Lin CC, Chiu HJ, Chen JY, Liou YJ, Wang YC, Chen TT, Bei YM. Switching from clozapine to zotepine in patients with schizophrenia: a 12-week prospective, randomized, rater blind, and parallel study. J Clin Psychopharmacol. 2013;33(2):211-4. doi: 10.1097/JCP.0b013e31828700c7.
-
Shrestha S, Agha RS, Khan Z, Shah K, Jain S. Considering loxapine instead of clozapine: A case series and literature review. Cureus. 2021;13(1):e12919.
-
Erley J, Goldsborough S, VandenBerg A, Audu A. Loxapine in patient with clozapine-resistant psychosis. Ment Health Clin. 2021;11(4):263-266. doi: 10.9740/mhc.2021.07.263.
-
Zink M, Henn FA, Thome J. Combination of amisulpride and olanzapine in treatment-resistant schizophrenic psychoses. Eur Psychiatry. 2004;19(1):56-8. doi: 10.1016/j.eurpsy.2003.09.002.
-
Hanisch F, Friedemann J, Pillmann F. Combined treatment with quetiapine and sertindole in therapy refractory insomnia after clozapine discontinuation.
-
J Psychopharmacol. 2010;24(11):1725-6. doi: 10.1177/0269881109348159.
-
Dunkley MJ, reveley MA. Successful treatment of refractory schizophrenia with combined olanzapine and quetiapine in a patient with a prolactin secreting pituitary microadenoma. J Psychopharmacol. 2005;19(1):97-101. doi: 10.1177/0269881105048903.
-
Citrome L, Kantrowitz JT. Olanzapine dosing above the licensed range is more efficacious than lower doses: fact or fiction? Expert Rev Neurother. 2009;9(7):1045-58. doi: 10.1586/ern.09.54.
-
Barnes TR, Drake R, Paton C, Cooper SJ, Deakin B, Ferrier IN, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: Updated recommendations from the British Association for Psychopharmacology.
-
J Psychopharmacol. 2020;34(1):3-78. doi: 10.1177/0269881119889296.
-
Zheng W, Xiang YT, Yang XH, Xiang YQ, de Leon J. Clozapine augmentation with antiepileptic drugs for treatment-resistant schizophrenia: A meta-analysis of randomized controlled trials. J Clin Psychiatry. 2017;78(5):e498-e505. doi: 10.4088/JCP.16r10782.
-
Tiihonen J, Wahlbeck K, Kiviniemi V. The efficacy of lamotrigine in clozapine-resistant schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2009;109(1-3):10-4. doi: 10.1016/j.schres.2009.01.002.
-
Small JG, Klapper MH, Malloy FW, Steadman TM. Tolerability and efficacy of clozapine combined with lithium in schizophrenia and schizoaffective disorder. J Clin Psychopharmacol. 2003;23(3):223-8. doi: 10.1097/01.jcp.0000084026.22282.5f.
-
Hahn MK, Remington G, Bois D, Cohn T. Topiramate augmentation in clozapine-treated patients with schizophrenia: clinical and metabolic effects. J Clin Psychopharmacol. 2010;30(6):706-10. doi: 10.1097/jcp.0b013e3181fab67d.
-
Muscatello MRA, Bruno A, Pandolfo G, Mico U, Bellinghieri PM, Scimeca G, et al. Topiramate augmentation of clozapine in schizophrenia: a double-blind, placebo-controlled study. J Psychopharmacology. 2011;25(5):667-74.
-
Kelly DL, Conley RR, Feldman S, Yu Y, McMahon RP, Richardson CM. Adjunct divalproex or lithium to clozapine in treatment-resistant schizophrenia. Psychiatry Q. 2006;77(1):81-95. doi: 10.1007/s11126-006-7963-9.
-
Demily C, Franck N. Gabapentin for ultra resistant schizophrenia with aggressive behavior. Schizophr Res. 2008;100(1-3):349-50.
-
Edinoff AN, Fort JM, Woo JJ, Causey CD, Burroughs CR, Cornett EM, Kaye AM, Kaye AD. Selective serotonin reuptake inhibitors and clozapine: Clinically relevant interactions and considerations. Neurol Int. 2021;13(3):445-463.
-
Szegedi A, Anghelescu I, Wiesner J, Schlegel S, Weigmann H, Härtter S, et al. Addition of low-dose fluvoxamine to low-dose clozapine monotherapy in schizophrenia: drug monitoring and tolerability data from a prospective clinical trial. Pharmacopsychiatry. 1999;32(4):148-54. doi: 10.1055/s-2007-979221.
-
Strous RD, Patel JK, Zimmet S, Green AI. Clozapine and paroxetine in the treatment of schizophrenia with obsessive-compulsive features. Am J Psychiatry. 1999;156(6):973a-974. https://doi.org/10.1176/ajp.156.6.973a.
-
Buchanan RW, Kirkpatrick B, Bryant N, Ball P, Breier A. Fluoxetine augmentation of clozapine treatment in patients with schizophrenia. Am J Psychiatry. 1996;153(12):1625-7. doi: 10.1176/ajp.153.12.1625.
-
Zoccali R, Muscatello MR, Cedro C, Neri P, la Torre D, Spina E, et al. The effect of mirtazapine augmentation of clozapine in the treatment of negative symptoms of schizophrenia: a double-blind, placebo-controlled study. Int Clin Psychopharmacol. 2004;19(2):71-6. doi: 10.1097/00004850-200403000-00003.
-
Mico U, Bruno A, Pandolfo G, Romeo VM, Mallamace D, Dárrigo C, et al. Duloxetine as adjunctive treatment to clozapine in patients with schizophrenia: a randomized, placebo-controlled trial. Int Clin Psychopharmacol. 2011;26(6):303-10. doi: 10.1097/YIC.0b013e32834bbc0d.
-
Szarmach J, Wlodarczyk A, Cubala WJ, Wiglusz MS. Benzodiazepines as adjunctive therapy in treatment refractory symptoms of schizophrenia. Psychiatria Danubiana. 2017;29(Suppl.3):349-352.
-
Grohmann R, Rüther E, Sassim N, Schmidt LG. Adverse effects of clozapine. Psychopharmacol (Berl). 1989;99(Suppl.):S101-4. doi: 10.1007/BF00442571.
-
Shim SS, Hammonds MD, Kee BS. Potentiation of the NMDA receptor in the treatment of schizophrenia: focused on the glycine site. Eur Arch Psychiatry Clin Neurosci. 2008;258(1):16-27. doi: 10.1007/s00406-007-0757-8.
-
Veerman SRT, Schulte PFJ, Smith JD, de Haan L. Memantine augmentation in clozapine-refractory schizophrenia: a randomized, double-blind, placebo-controlled crossover trial. Psychol Med. 2016;46(9):1909-21.
-
Kelly DL, Sullivan KM, McEvoy JP, McMahon RP, Wehring HJ, Gold JM, et al. Adjunctive minocycline in clozapine-treated schizophrenia patients with persistent symptoms. J Clin Psychopharmacol. 2015;35(4):374-81. doi: 10.1097/JCP.0000000000000345.
-
Stryjer R, Strous R, Bar F, Shaked G, Shiloh R, Rozencwaig S, et al. Donepezil augmentation of clozapine monotherapy in schizophrenia patients: a double blind cross-over study. Hum Psychopharmacol. 2004;19(5):343-6.
-
Doruk A, Uzun O, Ozşahin A. A placebo-controlled study of extract of ginkgo biloba added to clozapine in patients with treatment-resistant schizophrenia. Int Clin Psychopharmacol. 2008;23(4):223-7. doi: 10.1097/YIC.0b013e3282fcff2f.
Articole din ediţiile anterioare
Therapeutic options in ultra‑resistant schizophrenia. Nonpharmacological interventions (II)
The necessity to investigate clozapine-resistant schizophrenia (CRS) cannot be overemphasized due to the significant negative impact this pathology...