Toxoplasmosis is caused by a single-celled parasite called Toxoplasma gondii which is an obligate, intracellular, protozoan. T. gondii is found worldwide, being one of the most common parasites. Although these infections are usually asymptomatic, or associated with flu-like symptoms, occasionally they occur during the first few weeks following exposure. However, in infants, in HIV/AIDS patients and in other people with low immunity, the infection can cause a severe illness. Latent toxoplasmosis has been linked to several psychiatric disorders (e.g., schizophrenia, bipolar disorder, personality disorders) and to suicidal behavior. In pregnant women, the infection may cause severe sequelae in the infant, including mental retardation, blindness or epilepsy. Toxoplasmosis is considered one of the most neglected parasitic infections, being declared by Centers for Disease Control and Prevention (CDC) a public health problem. These infections are neglected because they receive relatively little attention for their surveillance, prevention and treatment.
Toxoplasmoza şi riscul apariţiei bolilor psihice
Toxoplasmosis and the risk for psychiatric disorders
First published: 24 septembrie 2019
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/Psih.58.3.2019.2524
Abstract
Rezumat
Toxoplasmoza este cauzată de un parazit unicelular, Toxoplasma gondii, obligatoriu intracelular, cu răspândire generală – unul dintre cei mai întâlniţi paraziţi. Infecţia cu acest parazit este de obicei asimptomatică sau se poate manifesta ca un sindrom pseudogripal în primele săptămâni după expunere. Totuşi, la copilul mic, persoanele cu infecţie HIV/SIDA sau la persoanele imunocompromise, infecţia poate cauza afecţiuni grave. Mai mult, toxoplasmoza latentă a fost asociată cu o serie de tulburări mintale şi de comportament (ex.: schizofrenia, tulburarea afectivă bipolară, tulburările de personalitate), cu comportamentul suicidar şi cu întârzierea în dezvoltarea mintală, cecitate şi epilepsie la fătul expus in utero. Cu toate acestea, resursele direcţionate către monitorizarea, prevenirea şi tratamentul toxoplasmozelor sunt relativ limitate, în ciuda faptului că au fost declarate ca reprezentând o problemă de sănătate publică de către Centrele de Control şi Prevenire a Bolilor din Statele Unite ale Americii (CDC).
Introduction
Toxoplasmosis is caused by a single-celled parasite called Toxoplasma gondii, which is an obligate, intracellular, protozoan.
T. gondii is found worldwide, being one of the most common parasites. Although these infections are usually asymptomatic, or associated with flu-like symptoms, they occasionally occur during the first few weeks following exposure. However, in infants, in HIV/AIDS patients and other people with low immunity, the infection can cause a severe illness. Although it is thought to be relatively harmless in immunocompetent adults, latent toxoplasmosis has been linked to several psychiatric disorders (e.g., schizophrenia, bipolar disorder, personality disorders) and with suicidal behavior(1). In pregnant women, infections may cause severe sequelae in the infant, including mental retardation, blindness or epilepsy.
Toxoplasmosis is considered one of the most neglected parasitic infections, being declared by Centers for Disease Control and Prevention (CDC) a public health problem. These infections are neglected because they receive relatively little attention for their surveillance, prevention and treatment.
Biology, life cycle and transmisson
T. gondii life cycle has three stages: tachyzoite – in the acute stage of infection, invades and replicates in cells; bradyzoite – in latent infections, present in tissue cysts; and sporozoite – in oocyst formed after the sexual stage of life cycle, enviromentally resistant(2). Tachyzoites invade and replicate within cells and are responsible for congenital toxoplasmosis. T. gondii tachyzoites invades and multiply in all organs, especially in muscles (including miocardial muscle), liver, spleen, lymph nodes and central nervous system. Cell invasion results in death of parasitized cell and in acute inflammatory reactions.
Humans acquire T. gondii through ingestion of undercooked meat, contact with feline feces and, rarely, through drinking contaminated water (Vancouver study, 1995) or through transplantation of contaminated organ(3), or from women who can transmit the infection to their unborn fetus transplacentally.
Epidemiology and risk factors
Human infection is acquired by ingestion of tissue cyst in raw, poorly cooked or cured meat, lamb and pork, or by ingestion of sporocyst derived from cat feces contaminating soil or vegetables, and also through unfiltered water or organ transplantation.
The difference of T. gondii prevalence in different countries has been associated with diet, climate and cat contact, therefore Toxoplasma infections apparently are most common in warm, wet areas with large cat population where meat is eaten lightly cooked or raw. Recent studies indicate that the relative importance of these routes of transmission may vary by region. The centres involved in the study – Naples, Lausanne, Copenhagen, Oslo, Brussels and Milan – operate a screening for toxoplasmosis. In five centres, women were prospectively identified by prenatal screening(4,5). A decline in the prevalence of the disease has been noted recently in many developed countries(4). The prevalence of previous Toxoplasma infection in pregnant women ranges from 10% in the United Kingdom(6) and Norway(7) to around 55% in France(8) and Greece(9); in many countries, it has declined sharply over the past three decades(10-12).
There is little information regarding T. gondii prevalence in the general human population in Romania(13). A recent study in western Romania demonstrated a high prevalence of T. gondii antibodies (64.8%)(14). The decline in the prevalence can be associated with freezing meat and the introduction of intensive farming techniques which separate cats from livestock(4).
In Romania, in Timiş county, the presence of T. gondii antibodies was demonstrated in 57.6% of the women of childbearing age who evaluate the prevalence in group studies. The results suggest a high prevalence of T. gondii antibodies among women of childbearing age(15).
The seroprevalence of women of reproductive age (16-49 years old) who had T. gondii antibodies is reported higher in Serbia (33%)(16), Croatia (38.1%)(17), Spain (43.8%)(18), Belgium (49%)(19), Hungary (69%)(20) and Turkey (60.4%)(21).
The overall risk of congenital infection from acute T. gondii infection during pregnancy is 20% to 50%(2).
There are studies that demonstrate a greater rate of T. gondii seropositive status in subjects with psychiatric disorders. The rate of T. gondii seropositive subjects in various studies was 15.9% or 14.1% in the United States of America(1,22). These findings are consistent with previous T. gondii seropositive status data, suggesting a relationship with self-directed aggression (i.e., suicidal behavior) and a relationship involving schizophrenia or mania – disorders in which many individuals are often aggressive(23,24).
Eating raw oysters, clams or mussels is a new risk factor for recent T. gondii infection(25). In addition to this new factor, there are others well known, such as: eating undercooked meat, especially pork, lamb, and wild-game meat (such as venison) and drinking unpasteurized goat’s milk(5,25-27). Another risk factors are associated with exposure to kittens. Kittens which have access to rodents and birds often become infected with T. gondii when they are weaned, grow older, and develop hunting skills. Via their feces, a litter of kittens could contaminate the environment with oocysts, which could survive for months or years in the soil. To avoid environmental contamination, a litter box should be used and feces cleaned up daily, preferably by a non-pregnant, non-immunosuppressed person(25,27,28). The daily cleaning of the litter box helps prevent T. gondii infection because the organism requires one or more days to sporulate and become infectious after being shed in the feces(27). The soil-related risk factors (e.g., gardening, washing fruits and vegetables) significantly increased the risk of infection with T. gondii.
Congenital infection
Unfortunately, in many eastern European countries and in Asia, it has been assumed that chronic T. gondii infection is a common cause of infertility and abortion(13).
Acute maternal infection leads to the hematogenous propagation through the placenta. Overall, the risk of transmission is 30% and increases with the date of maternal infection, from less than 15% at 13 weeks of gestation to almost 71% at 36 weeks(29).
Congenital T. gondii infection may occur in one of four forms: (1) a neonatal disease; (2) a disease (severe or mild) occurring in the first months of life; (3) sequelae or relapse of a previously undiagnosed infection during infancy, childhood or adolescence; or (4) a subclinical infection(30).
Congenital toxoplasmosis causes a wide spectrum of clinical manifestations, from no symptoms at birth to severe clinical symptoms. The most severe clinical findings observed in affected infants include chorioretinitis, cerebral calcifications, hydrocephalus, pneumonia and disseminated disease(31). A European study (Syrocot, 2007)(32) estimated the incidence of brain lesions to be 30% after maternal infection at 5 weeks of gestation, 10% at 20 weeks of gestation, and less then 5% after third trimester infection(29). Retinochoroiditis is the most frequent complication of congenital toxoplasmosis. The lesions can occur any time in childhood and as early adulthood, and can be associated with poor visual acuity or even blindness.
The treatment of the mother during pregnancy is an attempt to reduce the frequency and severity of fetal infection. Spiramycin has been estimated to reduce the incidence of vertical transmission by about 60%(33).
In a recent study of congenital toxoplasmosis performed in 2011 by Olariu et al. in United States, one or more severe clinical manifestations of congenital toxoplasmosis were reported in 84% of the infants, and included eye disease (92.2%), brain calcifications (79.6%) and hydrocephalus (67.7%). In 61.6% of the infants, eye disease, brain calcifications and hydrocephalus were present concurrently. T. gondii-specific IgM, IgA, and IgE antibodies were present in 86.6%, 77.4% and 40.2% of the infants, respectively(31).
In South America, recent studies demonstrated that relatively more children had retinochoroiditis during the first year in Brazil than in Europe (50% versus 10%). The children in Brazil had larger lesions, which were more likely to be multiple and to affect the posterior pole. In Brazil, the visual impairment was predicted for most affected eyes (87%), but not in Europe (29%) – Table 1(34).
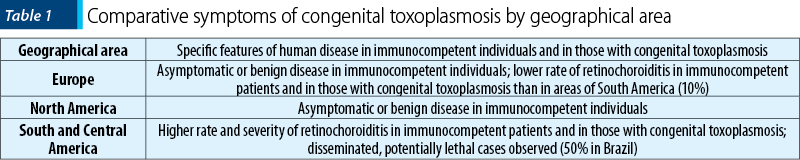
T. gondii causes more severe ocular disease in congenitally infected children in Brazil compared to Europe. The marked differences in the frequency, size and multiplicity of retinochoroidal lesions may be due to infection with more virulent genotypes of the parasite that predominate in Brazil, but are rarely found in Europe(34).
The congenital toxoplasmosis in South America is more symptomatic than in Europe, as demonstrated by two different reports comparing cohorts of congenitally infected children from different continents. The higher severity of South American cases was an unexpected result of the Systematic Review on Congenital Toxoplasmosis (SYROCOT) international collaborative study(32). For this analysis, 25 cohorts of infected mothers from Europe, North America and South America, identified during prenatal screening, were selected. The risk of ocular lesions was much higher among Colombian and Brazilian children (47%; 18/38) than among European children (14%; 79/550); the crude risk of intracranial lesions was also much higher among children from South America (53% 20/38) than among those from Europe (9%; 49/550)(32). Additionally, a comparative prospective cohort study of congenitally infected children in Brazil and Europe found that Brazilian children had eye lesions that were larger, more numerous and more likely to affect the part of the retina responsible for central vision, compared with their counterparts in Europe. More children developed retinochoroiditis sooner in Brazil than in Europe, and retinochoroidal lesion recurred at an earlier age in Brazil than in Europe(35). By 4 years of age, the probability of a second lesion among children with a first lesion was 43% in Brazil compared with 29% in Europe, and the risk of multiple recurrences was also greater in Brazil(36). Moreover, a recent report about 178 congenitally infected children in the southeastern region of Brazil found a high rate of early retinochoroidal involvement (80%) and 47% of them had active lesions during the first 3 months of life(36). Brazil is not the only nation that is now reporting the clinical characteristics of the congenital ocular disease in South America. A study in Colombia found that toxoplasmosis was the second commonest cause of congenital blindness(37).
Diagnosis
The diagnosis of acute infection with T. gondii may be established by the isolation of the organism from blood or body fluids, the demonstration of the presence of cysts in the placenta or tissues of a fetus or newborn, the demonstration of the presence of antigen or organisms or both in sections or preparations of tissues and body fluids, the demonstration of antigenemia and antigenin serum and body fluids, specific nucleic acid sequences or serologic test (e.g., ELISA – IgM and IgG T. gondii antibodies)(30).
The demonstration of tachyzoites in tissues (e.g., brain biopsy, bone marrow aspirate) or body fluids (ventricular fluid or cerebrospinal fluid, aqueous humor, sputum) establishes the diagnosis of active toxoplasmosis. Sabin-Feldman dye test is a complement-lysis-based assay and relatively sensitive and specific for anti-Toxoplasma IgG antibody. The test is considered more reliable than available ELISA kits(38,39).
In infants with neurological disorder, anti-Toxoplasma IgM and IgA antibodies plus cerebrospinal fluid PCR to detect Toxoplasma gondii DNA are considered to provide a high sensitivity for the diagnosis of congenital toxoplasmosis(39,40).
Additionally, Western Blot analysis is used to detect IgM and IgA(39,41), and RT-PCR for DNA in amniotic fluid with 98% sensitivity and 100% specificity(39,42).
Treatment
We recommend specific therapy in every case of congenital toxoplasmosis or congenital T. gondii infection in infants younger than 1 year of age. Insufficient data are available to allow the proper evaluation of treatment in the asymptomatic infected infant(30).
The evaluation of treatment efficacy of congenital T. gondii infection is difficult due to the high morbidity (both early and late) and mortality rates associated with this congenital infection(30). The evaluation of treatment is difficult because of the variations in severity and outcome of the infection and the disease. The agents that can be recommended for specific therapy at present are beneficial against the tachyzoite form, but none has been shown to effectively eradicate the encysted form, especially from the central nervous system (CNS) and eye(30).
In congenital toxoplasmosis, the treatment is made with: pyrimethamine in dosage of 1 mg/kg every 12 hours for two days, then 1 mg/kg daily for 2-6 months, then this dose every Monday, Wednesday, Friday for one year plus sulfadiazine 50 mg/kg every 12 hours, for one year, plus folinic acid 10 mg 3 times weekly during and for one week after pyrimethamine therapy(43).
Spiramycin has an antibacterial spectrum comparable to that of erythromycin and is active against T. gondii. Spiramycin is supplied as syrup and in capsules. The usual daily dose in adults is 1 g, three times a day, or a daily regimen of 2 g by mouth and a dosage schedule of 3 g daily(43).
Atovaquone is the most promising new drug available, but is not yet approved for use in pregnant women and small children(44).
Association with psychiatric disorders
Evidence in the literature suggests that Toxoplasma gondii can modify the behavior of humans, increasing its impact on public health. T. gondii can affect the brain both in the acute phase and in the chronic or latent forms of the infection. It was shown that behavioral disorders are mainly related to latent forms of infection(45). Some studies reported that in this form of infection, T. gondii causes neurological and behavioral effects in humans(46). Toxoplasmosis, despite being relatively harmless in immunocompetent adults, in its latent form has been associated with several neuropsychological disorders and with suicidal behavior(1).
The biological plausibility underlying the association of T. gondii infection with neuronal disruption is as follows: the influence on the synthesis of dopamine and other neurotransmitters, through the synthesis of homologous proteins; inflammatory response in the brain due to the presence of the parasite; stimulation of the immune system with production of antibodies against autoantigens; and increase in the synthesis and secretion of proinflammatory cytokines(47,48). The behavioral changes produced by lesions in the brain tissue caused by T. gondii may also be related with the location of the parasite in specific regions of the brain(48,49). Few studies have described the tropism of the parasite in different regions of the brain, but reactivation studies in immunocompromised individuals showed that different regions of the brain may be affected(48,50,51).
Whether T. gondii infection induces personality changes or certain personality factors influence the likelihood of becoming infected with the parasite remains unclear(48).
In the CNS, Toxoplasma has a selective tropism for neuronal cells over resident glial cells, and establishes a latent chronic infection by differentiating into encysted bradyzoites(52).
Emerging studies now posit the presence of Toxoplasma as an environmental factor that can either act as a trigger for disease onset, a factor that can exacerbate the disease condition, or sometimes even ameliorate or halt disease progression. The strongest link is perhaps between Toxoplasma and the susceptibility of individuals towards the development of schizofrenia (SZ) and bipolar disorder (BD), and recent reviews have discussed this possible connection(53-59). Schizofrenia, affecting about 1% of the world population(60), has a major socioeconomic impact worldwide, and studies have consistently shown that the prevalence of Toxoplasma infection is unusually high in SZ patients(61). Toxoplasma infection is not only correlated with the appearance of SZ(59,62-64), but the effect of latent toxoplasmosis on the risk of developing SZ is stronger than that of any SZ-associated gene variant identified in genome-wide analyses(52,65).
There have been some association studies linking Toxoplasma infection to depression(66,67) and epilepsy(68-70).
The infection was also correlated with depression in pregnant women(71). The effects of maternal immune activation on offspring mental health is a widely studied field of research(72,73), and thus it is no surprise that Toxoplasma infection in utero is associated with a high risk of developing psychiatric diseases, by affecting fetal brain development and increasing the vulnerability to develop SZ later in life(52,55,74-76).
Apart from the development of neuropsychiatric disorders in offspring, maternal infection with Toxoplasma causes spontenous abortions(2,77,78) and lower body weight at birth(78), and is associated with harm to the developing fetus due to an increased risk of self-directed violence in pregnant females(79).
Latent toxoplasmosis, the asymptomatic persistence of cysts in host tissues, including the brain, is prevalent in 25-30% of the global population(3), with a relatively lower prevalence (10-15%) reported in the US(26). Though previously considered harmless in immune-competent adults, latent toxoplasmosis has recently been associated with suicidal self-directed violence (SSDV), although the mechanisms underlying this association are not fully understood(79-86).
The number of articles that reported on the possible association of chronic and latent T. gondii infection in humans with behavioral changes is relatively scarce; however, some evidence showed the relationship of this infectious agent with the occurrence of neuropathological disorders, especially in the psychiatric sphere, such as schizophrenia(48).
Prevention
Educational materials containing messages on how to prevent pregnant women from becoming infected have resulted in reduced rates of seroconversion. Educational measures should be in written form (e.g., books, magazines, or simple handouts), available in different languages, and integrated into existing prenatal programs, visits and classes. Ultimately, it is the responsibility of health care policy makers and physicians to educate both pregnant women and women who are considering becoming pregnant, with regard to preventive measures. Most important is to inform these women that all meat should be prepared “well done” (not “pink” in the center). Meat should be heated throughout to at least 67°C (153°F). Freezing to at least –20°C (4°F) for 24 hours and thawing also kill T. gondii cysts(87-89).
Many countries have introduced educational programmes aimed at reducing the incidence of congenital toxoplasmosis(90,91).
The development of an effective vaccine against T. gondii appears to be an achievable goal, as primary infection results in a life-long protection against the parasite(90,92). The most effective approach for vaccine development has been the use of non-virulent mutated strains of the parasite(90,93). Hundreds of studies have exploited the potential of recombinant antigens for immunization against T. gondii infection(90,92).
Conclusions
Due to its ubiquitous distribution and its high prevalence, T. gondii remains one of the most important zoonotic agents of high medical and veterinary importance. During recent years, considerable progress has been made in various aspects of the parasite biology, including characterization of novel parasite genotypes, evaluating the relative contribution of risk factors for human infection, unravelling novel mechanisms of parasite interaction with its host (cell), and determining parasite and host factors that regulate tissue cyst formation and persistence. However, further efforts are clearly needed to ultimately translate our increased knowledge into an improved health management of toxoplasmosis patients(94).
There is a strong relationship between suicidal behavior and impulsive aggressive behaviour(95), either as a dimension or as a category, and a recent study(86) reported that T. gondii seropositivity status may be associated with high self-reported trait aggression and impulsivity in mentally healthy individuals(1).
Having antibodies to Toxoplasma gondii, presumed evidence of past infection, was found to be an intermediate risk factor for the development of schizophrenia(96).
Since most primary infections during pregnancy are asymptomatic and the screening for primary infection is problematic, primary prevention is the most logical method to lower the risk of congenital infection. Both women of childbearing age and physicians need to be informed of the risks associated with undercooked meat and soil contact. There is also a need to educate women on the safe handling of cat litter during pregnancy(97).
Finally, more research needs to be done on the most effective means of primary prevention of toxoplasmosis through education(2).
Bibliografie
- Coccaro EF, Lee R, Groer MW, Can A, Coussons-Read M, Postolache TT. Toxoplasma gondii infection: relationship with aggression in psychiatric subjects. J Clin Psychiatry. 2016; 77(3):334-341.
- Jones JL, Lopez A, Wilson M, Schulkin J, Gibbs R. Congenital Toxoplasmosis: A Review. Obstetrical and Gynecological Survey. 2001; vol. 1, no. 5, 296-305.
- Pappas G, Roussos N, Falagas ME. Toxoplasmosis snapshots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int J Parasitol. 2009; 39:1385 94.
- Richard EH. Toxoplasmosis. In: Cook GC et al. Manson’s Tropical Disease, Elsevier Saunders, 22nd Ed. 2009; pp. 1367-1372.
- Cook AJ, Gilbert RE, Buffolano W. Sources of Toxoplasma infection in pregnant women: European multicentre case-control study. European Research Network on Congenital Toxoplasmosis. BMJ. 2000; 321:142–7.
- Allain JP, Palmer CR, Pearson G. Epidemiological study of latent and recent infection by Toxoplasma gondii in pregnant women from a regional population in the UK. J Infect. 1998; 36:18996.
- Jenum PA, Kapperud G, Stray Pedersen B, Melby KK, Eskild A, Eng J. Prevalence of Toxoplasma gondii specific immunoglobulin G antibodies among pregnant women in Norway. Epidemiol Infect. 1998; 120:8792.
- Ancelle T, Goulet V, Tirard Fleury V, Baril L, du Mazaubrun C, Thulliez P, et al. La Toxoplasmose chez la femme enceinte en France en 1995. Resultats d’une enquete nationale perinatale. Bulletin Epidemiologique Hebdomadaire. 1996; 51:2279.
- Decavalas G, Papapetropoulou M, Giannoulaki E, Tzigounis V, Kondakis XG. Prevalence of Toxoplasma gondii antibodies in gravidas and recently aborted women and study of risk factors. Eur J Epidemiol. 1990; 6:2236.
- Horion M, Thoumsin H, Senterre J, Lambotte R. 20 years of screening for toxoplasmosis in pregnant women. The Liege experience in 20,000 pregnancies. Rev Med Liege. 1990; 45:4927.
- Ades AE, Nokes DJ. Modeling age and timespecific incidence from seroprevalence: toxoplasmosis. Am J Epidemiol. 1993; 137:102234.
- Nokes DJ, Forsgren M, Gille E, Ljungstrom I. Modelling toxoplasma incidence from longitudinal seroprevalence in Stockholm, Sweden. Parasitology. 1993; 107:3340.
- Dubey JP, Hotea I, Olariu TR, Jones JL, Dărăbuş G. Epidemiological review of toxoplasmosis in humans and animals in Romania. Parasitology. 2014; 141:311–25;
- Olariu TR, Petrescu C, Dărăbuş G, Lighezan R, Creţu O. Seroprevalence of Toxoplasma gondii in Western Romania. Informa Healtcare Infectious Disease. 2015; Early Online, 1-4.
- Olariu TR, Dărăbuş G, Creţu O, Jurovits O, Giura E, Erdelean V, Marincu I, Iacobiciu I, Petrescu C, Koreck A. Prevalence of Toxoplasma gondii antibodies among women of childbearing age in Timiş County. Lucrări ştiintifice de medicină veterinară. 2008; Vol. XLI, 367-371;
- Bobic B, Nikolic A, Klun I, Vujanic M, Djurkovic-Djakovic O. Undercooked meat consumption remains the major riskfactor for Toxoplasma infection in Serbia. Parassitologia. 2007; 49: 227–30.
- Punda-Poli V, Tonkic M, Capkun V. Prevalence of antibodies to Toxoplasma gondii in the female population of the County of Split Dalmatia, Croatia. Eur J Epidemiol. 2000; 16 : 875–7;
- Pujol-Riqué M, Quintó L, Danés C, Valls ME, Coll O, Jiménez De Anta MT. Seroprevalence of toxoplasmosisin women of childbearing age, 1992-1999. Med Clin. 2000; 115: 375–6.
- Breugelmans M, Naessens A, Foulon W. Prevention of toxoplasmosis during pregnancy – an epidemiologic survey over 22 consecutive years. J. Perinat Med. 2004; 32: 211–14.
- Szénási Z, Ozsvár Z, Nagy E, Jeszenszky M, Szabó J, Gellén J, et al. Prevention of congenital toxoplasmosis in Szeged, Hungary. Int J Epidemiol. 1997; 26: 428–35.
- Harma M, Harma M, Gungen N, Demir N. Toxoplasmosisin pregnant women in Sanliurfa, Southeastern Anatolia City, Turkey. J. Egypt Soc Parasitol. 2004; 34: 519–25.
- Pearce BD, Kruszon-Moran D, Jones JL. The relationship between Toxoplasma gondii infection and mood disorders in the third National Health and Nutrition Survey. Biol Psychiatry. 2012; 72(4):290–295.
- Soyka M. Neurobiology of aggression and violence in schizophrenia. Schizophr Bull. 2011; 37(5):913–920.
- Ballester J, Goldstein T, Goldstein B, et al. Is bipolar disorder specifically associated with aggression? Bipolar Disord. 2012; 14(3):283–290.
- Jones JL, Dargelas V, Roberts J, Press C, Remington JS, Montoya JG. Risk factors for Toxoplasma gondii infection in the United States. Clin. Infect. Dis. 2009, 49:878–884.
- Dubey JP, Jones JL. Toxoplasma gondii infection in humans and animals in the United States. Int. J. Parasitol. 2008, 38:1257–1278.
- Lopez A, Dietz V, Wilson M, Navin TR, Jones JL. Preventing congenital toxoplasmosis. Centers for Disease Control and Prevention. MMWR Recomm Rep. 2000; 49(RR-2):59–68.
- Centers for Disease Control and Prevention. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009; 58(No. RR-4):1–207.
- Kieffer F, Wallon M. Congenital Toxoplasmosis. Handbook of Clinical Neurology. 2013; 112:1099-101.
- Remington JS, McLeod R, Thulliez P, Desmonts G. Toxoplasmosis. In: Remington JS, Klein J, editors. Infectious diseases of the fetus and new born infant. Elsevier Saunders; 2011; 918–1042.
- Olariu TR, Remington JS, McLeod R, Alam A, Montoya JG. Severe congenital toxoplasmosis in the United States – clinical and serological findings in untreated infants. The Peditaric Infectious Disease Journal. 2011; vol. 30, no. 12.
- SYROCOT (Systematic Review on Congenital Toxoplasmosis) Study Group. Effectiveness of prenatal treatment for congenital toxoplasmosis: a metaanalysis of individual patients’ data. Lancet. 2007; 369: 115–122.
- Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004; 363:1965–76.
- Gilbert RE, Freeman K, Lago EG, Bahia-Oliveira LMG, Tan HK, et al. Ocular Sequelae of Congenital Toxoplasmosis in Brazil Compared with Europe. PLoS Negl Trop Dis. 2008; 2(8): e277.
- Petersen E, Schmidt DR. Sulfadiazine and pyrimethamine in the postnatal treatment of congenital toxoplasmosis: what are the options? Expert Rev. Anti Infect. Ther. 2003; 1, 175–182.
- Vasconcelos-Santos DV, Machado Azevedo DO, Campos WR, et al. UFMG Congenital Toxoplasmosis Brazilian Group. Congenital toxoplasmosis in southeastern Brazil: results of early ophthalmologic examination of a large cohort of neonates. Ophthalmology. 2009; 116:2199–2205.
- de-la-Torre A, Gonzalez G, Diaz-Ramirez J, et al. Screening by ophthalmoscopy for toxoplasma retinochoroiditis in Colombia. Am J Ophthalmol. 2007; 143:354–356.
- Dando C, Gabriel KE, Remington JS, Parmley SF. Simple and efficient method for measuring anti-toxoplasma immunoglobulin antibodies in human sera using complement-mediated lysis of transgenic tachyzoites expressing beta-galactosidase. J. Clin. Microbiol. 2001; 39, 2122–2125.
- Oz HS. Maternal and Congenital toxoplasmosis, currently available and novel therapies in horizon. Front. Micriobiol. 2014; 5:385.
- Olariu TR, Remington JS, Montoya JG. Polymerase chain reaction in cerebrospinal fluid for the diagnosis of congenital toxoplasmosis. Pediatr. Infect. Dis. J. 2014; 33, 566–570.
- di Carlo P, Romano A, Casuccio A, Cillino S, Schimmenti MG, Mancuso G, et al. Investigation and management of Toxoplasma gondii infection in pregnancy and infancy: a prospective study. Acta Pharmacol. Sin. 2011; 32, 1063–1070.
- Teixeira LE, Kanunfre KA, Shimokawa PT, Targa LS, Rodrigues JC, Domingues W, et al. The performance of four molecular methods for the laboratory diagnosis of congenital toxoplasmosis in amniotic fluid samples. Rev. Soc. Bras Med. Trop. 2013; 46, 584–588.
- Hezard N, et al. Prenatal diagnosis of congenital toxoplasmosis in 261 pregnancies. Prenat. Diagn. 1997; 17, 1047–1054.
- Petersen E. Toxoplasmosis. Seminars in Fetal & Neonatal Medicines. 2007; 12:214:223.
- Fekadu A, Shibre T, Cleare AJ. Toxoplasmosis as a cause for behaviour disorders-overview of evidence and mechanisms. Folia Parasitol (Praha). 2010; 57(2):105–113.
- Dabritz HA, Conrad PA. Cats and toxoplasma: implications for public health. Zoonoses Public Health. 2010; 57(1):34–52.
- Fabiani S, Pinto B, Bruschi F. Toxoplasmosis and neuropsychiatric diseases: can serological studies establish a clear relationship? Neurol Sci. 2013; 34:417–425.
- Martinez VO, et al. Toxoplasma gondii infection and behavioral outcomes in humans: a systematic review. Parasitology research. 2018; 117.10: 3059-3065.
- McConkey GA, Martin HL, Bristow GC, Webster JP. Toxoplasma gondii infection and behaviour – location, location, location? J Exp Biol. 2013; 216(1):113–119.
- Schroeder PC, Post MJD, Oschatz E, Stadler A, Bruce-Gregorios J, Thurnher MM. Analysis of the utility of diffusion-weighted MRI and apparent diffusion coefficient values in distinguishing central nervous system toxoplasmosis from lymphoma. Neuroradiology. 2006; 48(10):715–720.
- Suzuki K, Masuya M, Matsumoto T, Ito N, Ohishi K, Maeda M, Katayama N. High-intensity signals in the basal ganglia from gadolinium-enhanced T1-weighted MRI as an early change in toxoplasma encephalitis in an AIDS patient. J Infect Chemother. 2010; 16(2):135–138.
- Tyebji, Shiraz, et al. Toxoplasmosis: A pathway to neuropsychiatric disorders. Neuroscience & Biobehavioral Reviews. 2018.
- Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am. J. Psychiatry. 2010; 167, 261–280.
- Del Grande C, Galli L, Schiavi E, Dell’Osso L, Bruschi F. Is Toxoplasma gondii a Trigger of Bipolar Disorder? Pathogens. 2017; 6, 3.
- Elsheikha HM, Büsselberg D, Zhu XQ. The known and missing links between Toxoplasma gondii and schizophrenia. Metab. Brain Dis. 2016; 31, 749–759.
- Henriquez SA, Brett R, Alexander J, Pratt J, Roberts CW. Neuropsychiatric disease and Toxoplasma gondii infection. Neuroimmunomodulation. 2009; 16, 122–133.
- Kannan G, Pletnikov MV. Toxoplasma gondii and cognitive deficits in schizophrenia: an animal model perspective. Schizophr. Bull. 2012; 38, 1155–1161.
- Sutterland AL, Fond G, Kuin A, Koeter MWJ, Lutter R, van Gool T, Yolken R, Szoke A, Leboyer M, de Haan L. Beyond the association. Toxoplasma gondii in schizophrenia, bipolar disorder, and addiction: systematic review and meta-analysis. Acta Psychiatr. Scand. 2015; 132, 161–179.
- Yolken R, Dickerson F, Torrey F. Toxoplasma and schizophrenia. Parasite Immunol. 2009; 31, 706–715.
- Saha S, Chant D, Welham J, McGrath J. A systematic review of the prevalence of schizophrenia. PLoS Med. 2005; 2, e141.
- Torrey EF, Bartko JJ, Lun ZR, Yolken RH. Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophr. Bull. 2007; 33, 729–736.
- Ebadi M, Akhlaghi H, Zamani MM, Beheshti H, Abolhassani H, Ayadi A, Dezdar J, Mortazzavi SH, Karami GR, Izadi M. The correlation between Toxoplama gondii infection and schizophrenia: a comparative study with family members (Control group). Scimetr. 2014; 2, 1–5.
- Fuglewicz A, Piotrowski P, Stodolak A. Relationship between toxoplasmosis and schizophrenia: a review. Adv. Clin. Exp. Med. 2017; 26, 1033–1038.
- Torrey EF, Yolken RH. Toxoplasma gondii and schizophrenia. Emerg. Infect. Dis. 2003; 9, 1375–1380.
- Purcell SM, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009; 460, 748–752.
- Gale SD, Brown BL, Berrett A, Erickson LD, Hedges DW. Association between latent toxoplasmosis and major depression, generalised anxiety disorder and panic disorder in human adults. Folia Parasitol. (Praha). 2014; 61, 285–292.
- Pearce BD, Kruszon-Moran D, Jones JL. The relationship between Toxoplasma gondii infection and mood disorders in the third national health and nutrition survey. Biol. Psychiatry. 2012; 72, 290–295.
- Ngoungou EB, Bhalla D, Nzoghe A, Dardé M-L, Preux PM. Toxoplasmosis and epilepsy – systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2015 Feb 19; 9(2):e0003525.
- Palmer BS. Meta-analysis of three case controlled studies and an ecological study into the link between cryptogenic epilepsy and chronic toxoplasmosis infection. Seizure. 2007; 16, 657–663.
- de Bittencourt PR, Adamolekum B, Bharucha N, Carpio A, Cossío OH, Danesi MA, Dumas M, Meinardi H, Ordinario A, Senanayake N, Shakir R, Sotelo J. Epilepsy in the tropics: I. Epidemiology, socioeconomic risk factors, and etiology. Epilepsia. 1996; 37, 1121–1127.
- Nourollahpour Shiadeh M, Rostami A, Pearce BD, Gholipourmalekabadi M, Newport DJ, Danesh M, Mehravar S, Seyyedtabaei SJ. The correlation between Toxoplasma gondii infection and prenatal depression in pregnant women. Eur. J. Clin. Microbiol. Infect. Dis. 2016; 35, 1829–1835.
- Canetta SE, Brown AS. Prenatal infection, maternal immune activation, and risk for schizophrenia. Transl. Neurosci. 2012; 3, 320–327.
- Knuesel I, Chicha L, Britschgi M, Schobel SA, Bodmer M, Hellings JA, Toovey S, Prinssen EP. Maternal immune activation and abnormal brain development across CNS disorders. Nat. Rev. Neurol. 2014; 10, 643–660.
- Brown AS, Schaefer CA, Quesenberry CP, Liu L, Babulas VP, Susser ES. Maternal exposure to toxoplasmosis and risk of Schizophrenia in adult offspring. Am. J. Psychiatry. 2005; 162, 767–773.
- Mortensen PB, Nørgaard-Pedersen B, Waltoft BL, Sørensen TL, Hougaard D, Torrey EF, Yolken RH. Toxoplasma gondii as a risk factor for early-onset schizophrenia: analysis of filter paper blood samples obtained at birth. Biol. Psychiatry. 2007a; 61, 688–693.
- Mortensen PB, Nørgaard-Pedersen B, Waltoft BL, Sørensen TL, Hougaard D, Yolken RH. Early infections of Toxoplasma gondii and the later development of schizophrenia. Schizophr. Bull. 2007b; 33, 741–744.
- Giakoumelou S, Wheelhouse N, Cuschieri K, Entrican G, Howie SEM, Horne AW. The role of infection in miscarriage. Hum. Reprod. Update. 2016; 22, 116–133.
- Wong SY, Remington JS. Toxoplasmosis in pregnancy. Clin. Infect. Dis. 1994; 18, 853–861 quiz 862.
- Pedersen MG, Mortensen PB, Norgaard-Pedersen B, Postolache TT. Toxoplasma gondii infection and self-directed violence in mothers. Arch. Gen. Psychiatry. 2012; 69, 1123–1130.
- Arling TA, Yolken RH, Lapidus M, Langenberg P, Dickerson FB, Zimmerman SA, et al. Toxoplasma gondii antibody titers and history of suicide attempts in patients with recurrent mood disorders. J Nerv Ment Dis. 2009; 197:905e8.
- Ling VJ, Lester D, Mortensen PB, Langenberg PW, Postolache TT. Toxoplasma gondii seropositivity and suicide rates in women. J Nerv Ment Dis. 2011; 199:440e4.
- Okusaga O, Langenberg P, Sleemi A, Vaswani D, Giegling I, Hartmann AM, et al. Toxoplasma gondii antibody titers and history of suicide attempts in patients with schizophrenia. Schizophr Res. 2011; 133:150e5.
- Postolache TT, Cook TB. Is latent infection with Toxoplasma gondii a risk factor for suicidal behavior? Expert Rev Anti Infect Ther. 2013; 11:339e42.
- Yagmur F, Yazar S, Temel HO, Cavusoglu M. May Toxoplasma gondii increase suicide attempt-preliminary results in Turkish subjects? Forensic Sci Int. 2010; 199:15e7.
- Zhang Y, Traskman-Bendz L, Janelidze S, Langenberg P, Saleh A, Constantine N, et al. Toxoplasma gondii immunoglobulin G antibodies and nonfatal suicidal self-directed violence. J Clin Psychiatry. 2012b; 73:1069e76.
- Cook TB, Brenner LA, Cloninger CR, et al. “Latent” infection with Toxoplasma gondii: association with trait aggression and impulsivity in healthy adults. J Psychiatr Res. 2015; 60:87–94.
- Montoya JG, Remington JS. Management of Toxoplasma gondii infection during pregnancy. Clin Infect Dis. 2008; 47:554–66.
- Dubey JP, Lindsay DS, Speer CA. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin Microbiol Rev. 1998; 11:267–99.
- Dubey JP, Hill DE, Jones JL, et al. Prevalence of viable Toxoplasma gondii in beef, chicken, and pork from retail meat stores in the United States: risk assessment to consumers. J Parasitol. 2005; 91:1082–93.
- Saadatnia G, Golkar M. A review on human toxoplasmosis. Scand J Infect Dis. 2012; 44(11):805-14.
- Rorman E, Zamir CS, Rilkis I, Ben-David H. Congenital toxoplasmosis – prenatal aspects of Toxoplasma gondii infection. Reprod Toxicol. 2006; 21:458 –72.
- Jongert E, Roberts CW, Gargano N, Forster-Waldl E, Petersen E. Vaccines against Toxoplasma gondii: challengesand opportunities. Mem Inst Oswaldo Cruz. 2009; 104:252–66.
- Innes EA. Vaccination against Toxoplasma gondii: an increasing priority for collaborative research? Expert Rev Vaccines. 2010; 9:1117–9.
- Schlüter D, Däubener W, Schares G, Groß U, Pleyer U, Lüder C. Animals are key to human toxoplasmosis. Int J Med Microbiol. 2014; 304(7):917-29.
- McCloskey MS, Ben-Zeev D, Lee R, et al. Prevalence of suicidal and self-injurious behavior among subjects with intermittent explosive disorder. Psychiatry Res. 2008; 158(2):248–250.
- Torrey EF, Bartko JJ, Yolken RH. Toxoplasma gondii and other risk factors for schizophrenia: an update. Schizophr Bull. 2012; 38(3):642–647.
- Kravetz JD, Federman DG.Toxoplasmosis in pregnancy. Am J Med. 2005; 118(3):212-6.
Articole din ediţiile anterioare
Psihopatologia personalităţii artistice LGBT – în comorbiditate cu toxoplasmoza
Autopsiile noastre psihiatrice, într-o abordare biopsihosocială, cercetează psihopatologiile unui număr de şapte scriitori LGBTG reprezentativi, ...
Episod psihotic la un pacient cu sindrom antifosfolipidic – studiu de caz
Un pacient în vârstă de 22 de ani, fără antecedente psihiatrice, tratat în Spitalul Clinic „Prof. Dr. Al. Obregia”, Secţia Clinică Psihiatrie VI...