Caracteristicile neonatale în malformaţiile urologice congenitale – experienţa noastră
The neonatal characteristics of congenital defects of the kidney and urinary tract – our experience
Abstract
Introduction. Congenital defects of the kidney and urinary tract occur in the prenatal period and are responsible for 20-30% of all developmental malformations. The purpose of this study is to assess the risk factors for these intrauterine developmental disorders. Materials and method. We performed a prospective study between June 2016 and August 2021 that included newborns from the “Bucur” Maternity, Bucharest, who were diagnosed with congenital defects of the kidney and urinary tract. The study included 273 newborns divided into two groups: newborns with developing anomalies (n=144; 52.7% of all the subjects), and newborns without these anomalies (n=129; 47.3% of all the newborns). The malformations were divided into major and minor impairments. Results. Minor renal malformations were found in 5.56% of the newborns with anomalies (five males and three females). Also, 50% of these subjects were identified during pregnancy with a suspicion of minor renal anomalies. Moreover, 71.43% of the patients with this diagnosis had their mother living in urban area, and 37.5% of the patients with minor renal malformations came from a mother with diabetes. Another 37.5% of the newborns with minor renal defects had their mother with a history of oncologic diseases. Furthermore, 15.28% of the newborns from the group with development anomalies had major renal defects, 77.78% of them being born with a caesarean section and 77.27% of them being diagnosed during pregnancy. Conclusions. Major renal malformations are diagnosed in the prenatal period in a higher proportion than minor renal anomalies. Mothers with diabetes, oncological or renal diseases have a higher incidence of giving birth to an infant with urologic defects. Alcohol consumption can lead to major renal anomalies. Caesarean section is often required for infants with major renal anomalies. Male sex is associated with a higher incidence for minor renal anomalies.Keywords
congenital defectskidneyrenal anomaliescancerdiabetesantenatal careRezumat
Introducere. Defectele congenitale ale rinichilor şi tractului urinar apar în perioada prenatală şi sunt responsabile pentru 20-30% dintre toate malformaţiile de dezvoltare. Scopul acestui studiu este de a descrie factorii de risc pentru aceste tulburări de dezvoltare intrauterină. Materiale şi metodă. Am efectuat un studiu prospectiv în perioada iunie 2016 – august 2021 care a inclus nou-născuţi de la Maternitatea „Bucur”, Bucureşti, diagnosticaţi cu defecte congenitale ale rinichilor şi tractului urinar. Studiul a inclus 273 de nou-născuţi împărţiţi în două loturi: nou-născuţi cu anomalii de dezvoltare (n=144; 52,7% dintre toţi subiecţii) şi nou-născuţi fără astfel de anomalii (n=129; 47,3% dintre toţi nou-născuţii). Malformaţiile au fost împărţite în afecţiuni majore şi minore. Rezultate. Malformaţii renale minore au fost constatate la 5,56% dintre nou-născuţii cu anomalii (cinci băieţi şi trei fete). Cu suspiciunea de anomalii renale minore au fost identificaţi în timpul sarcinii 50% dintre aceşti pacienţi. 71,43% dintre cei cu acest diagnostic au mama din mediul urban. 37,5% dintre cei cu malformaţii renale minore provin dintr-o mamă cu diabet. Alţi 37,5% dintre nou-născuţii cu defecte renale minore provin dintr-o mamă cu antecedente de boli oncologice. 15,28% dintre nou-născuţii din grupul cu anomalii de dezvoltare au defecte renale majore, 77,78% dintre aceştia fiind născuţi prin operaţie cezariană şi 77,27% dintre ei fiind diagnosticaţi în timpul sarcinii. Concluzii. Malformaţiile renale majore sunt diagnosticate în perioada prenatală într-o proporţie mai mare decât cele minore. Mamele cu diabet, boli oncologice sau renale au o incidenţă mai mare de a da naştere unui copil cu defecte de dezvoltare ale aparatului urinar. Consumul de alcool poate duce la anomalii renale majore. Naşterea prin cezariană este adesea necesară pentru feţii cu malformaţii renale majore. Sexul masculin este asociat cu o incidenţă mai mare a anomaliilor renale minore.Cuvinte Cheie
defecte congenitalerinichianomalii renalecancerdiabetmonitorizare prenatalăIntroduction
Congenital defects of the kidney and urinary tract represent approximately 20-30% of all malformations identified in the prenatal period(1). These defects can be unilateral or bilateral, and sometimes different anomalies coexist in the same person. Several development processes are responsible for urologic defects, the most cited reasons in the literature being: the failure of nephron development (renal dysplasia, renal tubular dysgenesis, renal agenesis, some types of nephronophthisis)(2,3), environmental factors (prenatal exposure to teratogens), defects of embryonic migration of the kidneys (renal ectopy), and abnormalities of the developing urinary collecting system (ureteropelvic junction obstruction, duplication of the collecting systems). The rate of congenital anomalies of the kidney and urinary tract in live and stillborn infants is 0.3-1.6/1000. A family history of congenital renal defects (CRD), but also a maternal history of kidney disease, cancer or diabetes represent important risk factors for developing minor or major renal defects(4,5).
Materials and method
We performed a prospective study between June 2016 and August 2021 that included newborns from the “Bucur” Maternity, Bucharest, who were diagnosed with congenital defects of the kidney and urinary tract. The study included 273 newborns divided into two groups: newborns with anomalies (n=144; 52.7% of all the subjects) and newborns without anomalies (n=129; 47.3% of all the newborns).
Regarding the gestation age, the newborns were grouped as extremely premature (below 28 weeks), early premature (28-32 weeks), moderate premature (32-24 weeks), lately premature (34-36 weeks), and term newborns (37 weeks or more). The malformations were divided into major and minor anomalies.
The data were collected using the Excel program and were processed with SPSS 25 variant statistic program. A p value was considered statistically significant when p≤0.05.
Results
A percentage of 80.56% of newborns with malformations were born at term, much more than those without this type of pathological condition (61.24%). Similarly, 6.2% of infants without anomalies were extremely premature and only 0.69% of infants with intrauterine development anomalies were born below 28 weeks. It has been observed a significant statistical difference with Chi-square Test between the presence of development anomalies and gestational age, a higher percentage of newborns with a normal gestational age in the group of infants with malformations than in the group without these anomalies, but with other pathologic conditions (c2=16.386; p=0.003; Phi and V Crammer’s coefficients = 0.245).
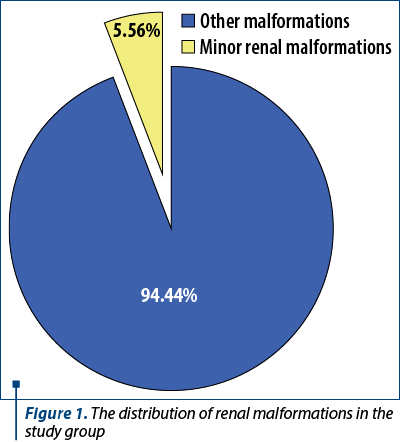
Minor renal malformations were found in 5.56% of the newborns with anomalies (Figure 1; five males and three females), all being born at term, half of them by a caesarean section. There were identified 50% of these patients during pregnancy with a suspicion of minor renal anomalies. Also, 71.43% of patients with this diagnosis had their mother living in urban areas.
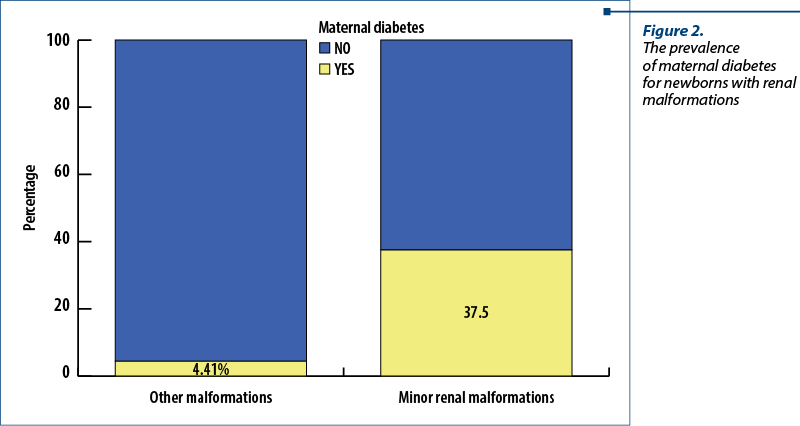
From our results, 37.5% of the patients with minor renal malformations and 4.41% of the newborns with other anomalies came from a mother with diabetes (Figure 2). A Chi-square test was applied to verify a possible significant statistical difference between the newborns with minor renal defects and those with other anomalies. The results were positive, the association between diabetes and minor renal anomalies being valid, with p<0.001 (c2=14.118; Phi and V Crammer’s coefficients = 0.313).
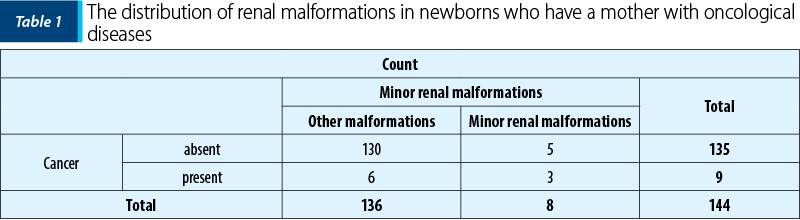
It has been found, from our data, that more newborns with minor renal defects (37.5%) than those with other development anomalies (4.41%) came from a mother with cancer in the past (Table 1). The association is moderate, with p<0.001 (c2=14.118, Phi and V Crammer’s coefficients = 0.313).
Referring to major renal defects, 15.28% of newborns from the group with development anomalies had this pathology. Most women who gave born to newborns with major renal defects came from urban area (88.24%). It has been proven a significant statistical difference between major renal anomalies and living in urban areas (c2=4.872; p=0.027). Almost all (90.9%) of newborns with major renal defects were born at term, 77.78% of them being born by a caesarean section. A significant part of major renal anomalies (77.27%) were diagnosed during pregnancy, this proportion being significantly higher than in other types of malformations (c2=20.743; p≤0.001).
In the group of newborns with renal anomalies, there were included two cases of in vitro fertilization (IVF). These two patients had a major renal anomaly, so we note IVF as a possible risk factor for major renal defects. Indeed, further analyses on more IVF cases are required to confirm a possible statistical association.
Although there is not a confirmed statistical association, we observed that women who gave birth to newborns with major renal anomalies (comparing with those who gave birth to infants with other anomalies) had a higher predisposition to drink alcohol (p=0.142). Moreover, 17.65% of mothers who have an infant with major renal defects were drinking alcohol, compared to 6.93% of them with this behavior, but with a newborn with another type of anomalies.
There was one case of major renal anomaly with a particular condition (the mother had also a kidney diseases). Although there is no association between renal malformations in newborns and mothers with kidney diseases, it is worth mentioning a possible risk of developing a major renal anomaly if the mother has a pathological kidney condition.
Discussion
Since the beginning of the 1990s, it has been recommended at least two ultrasound scans to identify different types of embryological malformations(6). The overall rate of identification for renal malformations is about 55% in most countries(7). This percentage is similar to the one from our study for minor renal anomalies, and the rate of detection for these with major renal development impairment was even higher. An important aspect for a lower identification rate for minor renal anomalies could be the fact that these defects are sometimes transient and other times they are getting worse(8). Thus, a better follow-up may increase the rate of detection for minor renal impairments.
A study conducted by Moore et al. on metropolitan Atlanta residents suggested that alcohol consumption in moderate doses during pregnancy may lead to renal agenesis or hypoplasia in infants(9). Our results suggest the same, more women who drink alcohol having a higher chance to give birth to an infant with major renal anomalies than those who do not consume alcohol.
Similar to Zhong-yi Li et al.(10), a higher prevalence of congenital anomalies of the kidney and urinary tract was described among infants whose mothers lived in urban areas. This can be attributed to a higher awareness of the antenatal care among women in urban areas, but also due to environmental exposures that could generate diverse malformations.
Banhidy et al. suggest an important association between type 1, type 2 or gestational diabetes and congenital urinary tract anomalies(11). Other studies concluded that maternal diabetes is a strong risk factor for renal agenesis(12,13). We also found a significant association between the presence of maternal diabetes and the risk of developing minor renal impairments.
A case-control analysis performed in Washington state by Shnorhavorian et al., using discharge records, showed that maternal renal disease was linked with a five-fold increase in the risk of kidneys anomalies, but also with a four-fold increase in the risk of ureter, bladder and urethra anomalies(5). From our data, we concluded that the infant with major renal anomaly could develop this condition due to the mother’s kidney disease.
Hays et al. reported a higher prevalence of CRD at lower gestational ages(14). In our study, it was found a higher prevalence of preterm births in the control group, possible due to other pathological conditions associated. A case-control study conducted in Taiwan by Tain You-Lin et al.(15), using national births registry, mentioned as risk factors for CRD the maternal gestational diabetes, maternal thalassemia/hemochromatosis, poly- or oligohydramnios, prematurity and being a boy. We also found a higher incidence of minor renal anomalies among the male sex. A higher incidence of caesarean section was observed for newborns with major renal anomalies than for those with minor renal defects. Polyhydramnios and oligohydramnios can be more severe if a major renal anomaly is present, so a caesarean section is often required for the safety of delivering.
The association between mothers with a personal history of oncologic diseases and CRD in infants was statistically demonstrated in our study, but the reason for this is still debated in medical literature. It is well known that radiotherapy and chemotherapy have a teratogenic effect in the first trimester(16,17). Other factors for generating CRD in infants who have mothers with oncological problems could be due to a similar mechanism of maternal thalassemia or hemochromatosis. A Japanese cohort study also described an increased risk for developing renal anomalies in infants whose mothers have oncological diseases(4).
Conclusions
Major renal anomalies are diagnosed in the prenatal period in a higher proportion than minor renal impairment. Alcohol consumption is a risk factor for major renal anomalies. Mothers living in urban areas and those who have diabetes or a personal history of oncological or renal diseases have a higher prevalence for infants with urologic anomalies. Caesarean section is more often required for infants with major renal defects and less needed for infants with minor impairments. Males have a higher incidence for minor renal anomalies than females.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
-
Queisser-Luft A, Stolz G, Wiesel A, et al. Malformations in newborn: results based on 30,940 infants and fetuses from the Mainz congenital birth defect monitoring system (1990-1998). Arch Gynecol Obstet. 2002;266(3):163-7.
-
Teeninga N, Kist-van Holthe JE, van den Akker EL, et al. Genetic and in vivo determinants of glucocorticoid sensitivity in relation to clinical outcome of childhood nephrotic syndrome. Kidney Int. 2014;85(6):1444-53.
-
Gribouval O, Gonzales M, Neuhaus T, et al. Mutations in genes in the renin-angiotensin system are associated with autosomal recessive renal tubular dysgenesis. Nat Genet. 2005;37(9):964-8.
-
Nishiyama K, Sanefuji M, Kurokawa M, et al. Maternal chronic disease and congenital anomalies of the kidney and urinary tract in offspring: a Japanese cohort study. Am J Kidney Dis. 2022;80(5):619-28.
-
Shnorhavorian M, Bittner R, Wright JL, Schwartz SM. Maternal risk factors for congenital urinary anomalies: results of a population-based case-control study. Urology. 2011;78(5):1156-61.
-
Sanders RC. In utero sonography of genitourinary anomalies. Urol Radiol. 1992;14(1):29–33.
-
Gloor JM, Ogburn Jr PL, Breckle RJ, Morgenstern BZ, Milliner DS. Urinary tract anomalies detected by prenatal ultrasound examination at Mayo Clinic Rochester. Mayo Clin Proc. 1995;70(6):526–33.
-
Vandervoort K, Lasky S, Sethna C, Frank R, Vento S, Choi-Rosen J, Goilav B, Trachtman H. Hydronephrosis in infants and children: natural history and risk factors for persistence in children followed by a medical service. Clin Med Pediatr. 2009;3:63-70.
-
Moore CA, Khoury MJ, Liu Y. Does light-to-moderate alcohol consumption during pregnancy increase the risk for renal anomalies among offspring? Pediatrics. 1997;99(4):E11.
-
Li ZY, Chen YM, Qiu LQ, et al. Prevalence, types, and malformations in congenital anomalies of the kidney and urinary tract in newborns: a retrospective hospital-based study. Ital J Pediatr. 2019;45(1):50.
-
Banhidy F, Acs N, Puho EH, et al. Congenital abnormalities in the offspring of pregnant women with type 1, type 2 and gestational diabetes mellitus: a population-based case-control study. Congenit Anom (Kyoto). 2010;50(2):115-21.
-
Davis EM, Peck JD, Thompson D, Wild RA, Langlois P. Maternal diabetes and renal agenesis/dysgenesis. Birth Defects Res A Clin Mol Teratol. 2010;88(9):722-7.
-
Parikh CR, McCall D, Engelman C, et al. Congenital renal agenesis: case-control analysis of birth characteristics. Am J Kidney Dis. 2002;39(4):689-94.
-
Hays T, Thompson MV, Bateman DA, et al. The prevalence and clinical significance of congenital anomalies of the kidney and urinary tract in preterm infants. JAMA Netw Open. 2022;5(9):e2231626.
-
Tain YL, Luh H, Lin CY, Hsu CN. Incidence and risks of congenital anomalies of kidney and urinary tract in newborns: a population-based case-control study in Taiwan. Medicine (Baltimore). 2016;95(5):e2659. Erratum in: Medicine (Baltimore). 2016;95(15):e8733.
-
Azim Jr HA, Peccatori FA, Pavlidis N. Treatment of the pregnant mother with cancer: a systematic review on the use of cytotoxic, endocrine, targeted agents and immunotherapy during pregnancy. Part I: solid tumors. Cancer Treat Rev. 2010;36(2):101-9.
-
Needleman S. Powell M. Radiation hazards in pregnancy and methods of prevention. Best Pract Res Clin Obstet Gynaecol. 2016;33:108-16.




