Carcinom endometrial mixt endometrioid şi neuroendocrin
Uterine carcinoma admixed with neuroendocrine carcinoma
Abstract
Neuroendocrine tumors of the genital tract are infrequent and most of them are located in the cervix or ovary. We present the case of a 54-year-old woman with endometrial carcinoma admixed with neuroendocrine tumor. Extensive histological assessments completed with additional immunohistochemical tests are necessary for the diagnosis of this rare entity.Keywords
neuroendocrine carcinomaendometrioid carcinomamixed tumorsRezumat
Tumorile neuroendocrine ale tractului genital sunt rare şi majoritatea sunt localizate în colul uterin sau ovar. Prezentăm cazul unei femei de 54 de ani cu carcinom endometrial amestecat cu tumoră neuroendocrină. Sunt necesare evaluări histologice extinse, completate cu teste imunohistochimice suplimentare, pentru diagnosticul acestei entităţi rare.Cuvinte Cheie
carcinom neuroendocrincarcinom endometrioidtumori mixteNeuroendocrine tumors are rarely found in the genital tract, making up approximately 2% of all gynecological tumors(1). In most cases, they appear on the cervix, but they can also be located on the ovary, uterus, vagina or vulva(2).
There is a wide spectrum of manifestations of tumor aggressiveness, correlated with the degree of tumor differentiation, thus explaining why some patients with well-differentiated tumors can survive for decades even in the metastatic stage, while patients with tumors at the opposite pole of this differentiation spectrum show survival rates below two years(3).
The most common neuroendocrine tumors of the genital tract are cervical carcinoma with small cell and ovarian carcinoid tumors(4), while neuroendocrine tumors of the uterus are extremely rare, making up less than 1% of the total number of uterine carcinomas(5).
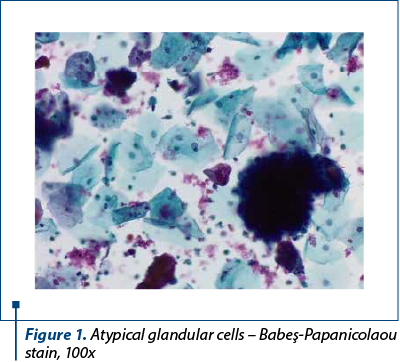
We present the case of a 54-year-old female patient who presented to our gynecological department with post-menopausal nonspecific symptoms (vaginal bleeding). The pelvic examination and ultrasound were performed, showing a tumor proliferation apparently invasive in the myometrium. A Babeş-Papanicolaou smear was performed, together with an endometrial biopsy. The Pap smear revealed the presence of atypical glandular cells (Figure 1) arranged in crowded overlapping groups, with a background with red blood cells.
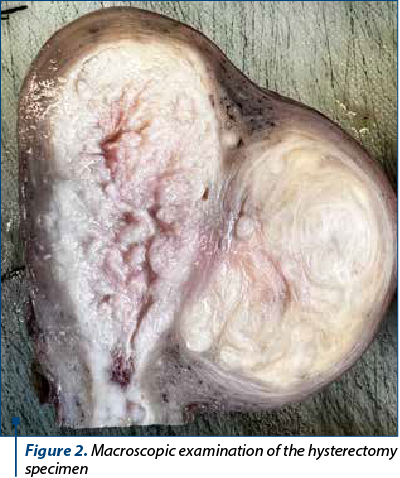
The endometrial biopsy showed pleomorphic cells with atypical nuclei, with disposition in small groups, suggestive for endometrial carcinoma. A surgical intervention was decided, based on the histological and imaging data. The hysterectomy specimen showed grey-whitish proliferation with hemorrhagic areas, friable, infiltrating over half the thickness of the wall, the minimal distance to the serosa being 0.7 cm, and an intramural leiomyoma (Figure 2).
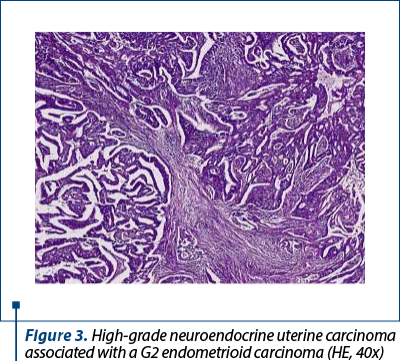
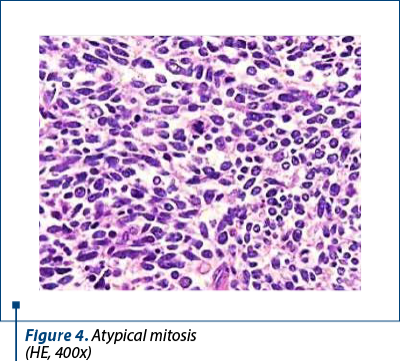
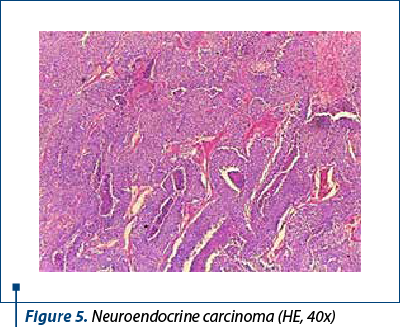
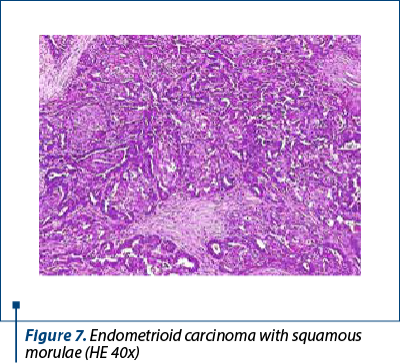
Histologically, we identified two tumoral populations (Figure 3), one composed of cells with nuclear pleomorphism, atypical mitoses (Figure 4), conspicuous nucleoli, arranged in islands (Figure 5), with areas of central necrosis (Figure 6), polymorphic inflammatory infiltrate, with the appearance of a poorly differentiated/undifferentiated carcinoma, and a second one, with cells with nuclear pleomorphism, arranged in glandular structures with area of squamous metaplasia, with the appearance of endometrioid adenocarcinoma (Figure 7).

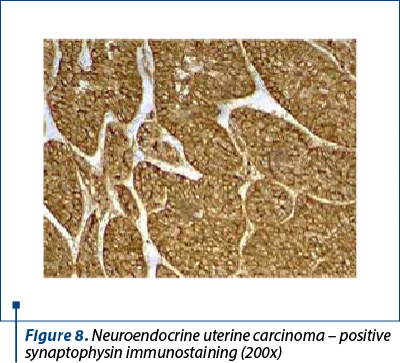
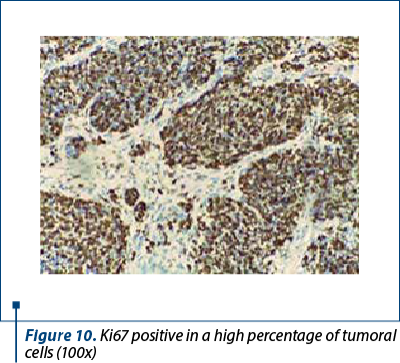
Immunohistochemistry was performed on the resection specimen. Cytokeratin 7 revealed focally positive tumor cells in one of the tumoral populations, the one with histological criteria of endometrioid carcinoma, while the other tumoral population was positive for synaptophysin and CD56, and negative for chromogranin and TTF1. Estrogen receptors were negative in the poorly differentiated carcinoma and positive in the endometrioid carcinoma. Protein p53 showed zonal positivity (mutation type pattern), CD10 was positive in isolated tumor cells and squamous morulae. Ki67 index was positive 80% in the area of poorly differentiated carcinoma and 40% in the endometrioid carcinoma (Figures 8, 9 and 10).

Positive immunolabeling for CD56 is not sufficient and is nonspecific, therefore, in the absence of another neuroendocrine marker, the diagnosis of neuroendocrine tumor should be reassessed. However, if the histological appearance is clearly neuroendocrine, the diagnosis is made even in the absence of positive immunohistochemistry tests. CK7 expressed positivity membrane in adenocarcinoma type tumor cells and only focally in the neuroendocrine component.
The differential diagnosis included dedifferentiated uterine carcinoma and collision tumor of endometrial adenocarcinoma and secondary dissemination of neuroendocrine carcinoma, but the patient did not present another neuroendocrine tumor, so the secondary character could not be established. The final diagnosis was mixed endometrioid and neuroendocrine carcinoma.
This type of tumors originate from the endometrium and divide into poorly differentiated carcinomas with small cell neuroendocrine, and poorly differentiated neuroendocrine carcinomas with large cells(6). On MRI, these tumors look like any endometrial cancer, with low-moderate signal in T2 and relative hypointensity to myometrial hyperintensity on sequences of dynamic contrast. However, when a highly aggressive endometrial tumor is found, the possibility of a neuroendocrine tumor should be included(7).
To classify a tumor as neuroendocrine, it must express at least two neuroendocrine markers such as chromogranin A, synaptophysin, CD56 or neuronal specific enolase. Numerous other peptides and hormones can also be identified, such as calcitonin, gastrin, serotonin, substance P, vasoactive intestinal peptide, pancreatic polypeptide, somatostatin and adrenocorticotropic hormone(8). The clinical picture of genital neuroendocrine tumors depends on the location tumors and is nonspecific. However, some patients may present with paraneoplastic syndrome due to this production of numerous peptides and hormones. The absence of clinical endocrine syndromes in most patients with neuroendocrine tumors can be explained by the release of an insufficient quantity of hormones(9).
Some tumors show a combination of neuroendocrine and non-neuroendocrine characteristics, mostly either glandular or squamous components. The percentage of neuroendocrine component within the tumor can start from a single cell neuroendocrine to an entire well-identifiable cell population. Thus, there are two terms that have been proposed: mixed non-endocrine endocrine carcinoma, and adenocarcinoma with neuroendocrine focal differentiation.
In literature, for the diagnosis of a mixed tumor, the identification of at least two major diagnostic parameters is recommended: a minimum 30% extension of each component and identification of neuroendocrine structural characteristics such as solid, diffuse growth patterns or well-differentiated areas(4). These criteria are highly subjective as there is no standardized definition of what 30% means in practice, the last WHO classification(10) recommending just to state the percentage of each individual component.
Carcinomas with focal neuroendocrine differentiation have been identified in numerous non-neuroendocrine tumors of the genital tract, such as endometrioid endometrial adenocarcinoma, endocervical carcinoma, and endometrioid or serous subtypes of ovarian carcinomas. These tumors contain less than a third of neuroendocrine cells which otherwise do not appear to influence prognosis.
Conclusions
The process of diagnosing genital neuroendocrine tumors is a complex one and often difficult, especially in the context of their low incidence and clinical data, along with non-specific paraclinical features. For these reasons, the histopathological examination plays a very important role, completed by immunohistochemical tests. No marker has yet been identified to confirm the genital origin, therefore extensive investigation of other organs is necessary. This pathology requires a multidisciplinary approach in order to ensure a treatment customized according to staging, the patient’s age, her desire to keep her reproductive function, but also the histological and immunohistochemical characteristics of the identified tumor. It is also very important to carry out a careful follow-up, since the rate of recurrence is high.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
-
Chun YK. Neuroendocrine tumors of the female reproductive tract: a literature review. J Pathol Transl Med. 2015;49(6):450–61.
-
Cree IA, White VA, Indave BI, Lokuhetty D. Revising the WHO classification: female genital tract tumours. Histopathology. 2020;76(1):151–6.
-
Reed NS. Neuroendocrine tumours of the gynecological tract. Curr Opin Oncol. 2016;28(5):412–8.
-
Volante M, Rindi G, Papotti M. The grey zone between pure (neuro)endocrine and non-(neuro)endocrine tumours: A comment on concepts and classification of mixed exocrine- endocrine neoplasms. Virchows Archiv. 2006;449(5):499–506.
-
Nguyen MLT, Han L, Minors AM, Bentley-Hibbert S, Pradhan TS, Pua TL, et al. Rare large cell neuroendocrine tumor of the endometrium: A case report and review of the literature. Int J Surg Case Rep. 2013;4(8):651–5.
-
Tu YA, Chen YL, Lin MC, Chen CA, Cheng WF. Large cell neuroendocrine carcinoma of the endometrium: A case report and literature review. Taiwanese J Obstet Gynecol. 2018;57(1):144-9.
-
Lopes Dias J, Cunha TM, Gomes FV, Callé C, Félix A. Neuroendocrine tumours of the female genital tract: a case-based imaging review with pathological correlation. Insights into Imaging. 2015;6(1):43–52.
-
Gardner GJ, Reidy-Lagunes D, Gehrig PA. Neuroendocrine tumors of the gynecological tract: A Society of Gynecologic Oncology (SGO) clinical document. Gynecol Oncol. 2011;122(1):190–8.
-
Bermúdez A, Vighi S, García A, Sardi J. Neuroendocrine cervical carcinoma: Adiagnostic and therapeutic challenge. Gynecol Oncol. 2001;82(1):32–9.
-
WHO Classification of Tumours of the Female Reproductive Organs – IARC; 5th ed, Lyon, France. 2020;459-60.





