Synchronous endometrial and ovarian carcinoma, endometrioid type – a pathologist’s insight. Case report
Carcinomul sincron endometrial şi ovarian de tip endometrioid – perspectiva anatomopatologului. Prezentare de caz
Abstract
Synchronous endometrial and ovarian carcinomas (SEOC) present a rare yet clinically significant occurrence, prompting challenges in diagnosis and management due to their heterogeneity in histological subtypes and molecular profiles. Diagnostic criteria, morphological features and the role of molecular analysis in differentiating between synchronous primaries and metastatic disease are discussed. Further investigation into predictive biomarkers and innovative therapies is crucial to enhance outcomes for these dual malignancies. The integration of molecular profiling, while promising, remains inconclusive in differentiating between independent primary tumors and metastatic disease, highlighting the need for further research and larger-scale studies in this field.Keywords
synchronous endometrial and ovarian carcinomasSEOCdiagnosismultidisciplinary approachhistological subtypesmorphological featuresRezumat
Carcinomul sincron endometrial şi ovarian (SEOC) este o entitate rară, dar semnificativă clinic, reprezentând o provocare de diagnostic şi de management, din cauza eterogenităţii, a subtipurilor histologice şi a profilului molecular. Criteriile de diagnostic histopatologic şi analizele de biologie moleculară sunt importante pentru diferenţierea dintre carcinomul primar şi boala metastatică. Testarea biomarkerilor de predicţie şi terapiile inovatoare au un rol esenţial în îmbunătăţirea prognosticului acestor patologii sincrone. Integrarea profilului molecular, deşi promiţătoare, în prezent nu poate diferenţia între tumorile primare independente şi boala metastatică, evidenţiind necesitatea unor cercetări suplimentare în acest domeniu.Cuvinte Cheie
carcinom endometrial şi ovarian sincroneSEOCdiagnosticabordare multidisciplinarăsubtipuri histologiceaspecte morfologiceIntroduction
Synchronous endometrial and ovarian carcinomas (SEOC) represent a relatively rare yet clinically significant occurrence where malignant tumors arise concurrently in the endometrium and ovaries. These synchronous tumors account for 50-70% of all synchronous female genital tract tumors(1), posing diagnostic complexity and therapeutic challenges due to their heterogeneity in histological subtypes and molecular profiles.
The criteria for diagnosing synchronous endometrial and ovarian carcinomas (SEOCs) take into account the histological similarity, the size, the presence of precursor lesions, location and invasion patterns(2).
While some efforts have been made by various researchers to introduce molecular analysis methods for precise SEOC diagnosis, a consensus has yet to be achieved. Distinguishing between endometrioid SEOC and metastatic cancer (either from endometrium to ovary, or vice versa) can pose even more challenges, as the histological features are similar. However, making this distinction is crucial in determining the most appropriate treatment strategy. Misdiagnosing SEOCs as FIGO stage III endometrial cancer (EC) or FIGO stage II ovarian cancer (OC) is not uncommon due to the rarity of SEOCs(3).
Case report
A 48-year-old woman presented to our department of gynecology with a history of abdominal discomfort and irregular vaginal bleeding spanning three months. Her medical record indicated no significant health concern. The physical examination revealed the presence of a palpable pelvic mass. The initial investigations involved a transvaginal ultrasound which revealed an enlarged uterus displaying heterogeneous echotexture alongside bilateral complex ovarian masses. Subsequent imaging by computed tomography (CT) validated the existence of a substantial endometrial mass alongside bilateral ovarian masses, raising the suspicion of malignancy. On inspection, the uterus appeared enlarged, measuring approximately 5.3x6x4.5 cm, displaying a polypoid formation of 1x1.2 cm, appearing white and friable on sectioning. The ovaries exhibited measurements of 2x1.5 cm and 4x2 cm, presenting cystic areas with white nodular regions. The confirmation of malignancy during the frozen section pathological examination of the ovaries prompted a comprehensive surgical intervention, including total abdominal hysterectomy, bilateral salpingo-oophorectomy, bilateral pelvic lymphadenectomy, and omentectomy. The analysis of peritoneal fluid confirmed the malignancy. The patient experienced an uneventful postoperative recovery. The histopathological examination of the excised specimens revealed the presence of endometrial and bilateral ovarian endometrioid carcinomas, both classified as histological grade 2. The endometrial component exhibited invasion limited to less than the inner half of the myometrium. Additionally, islands of adenomyosis were associated. Both ovaries displayed infiltration by endometrioid carcinoma, featuring squamous morules, along with accompanying endometriosis lesions (Figure 1). No signs of lymphovascular invasion were identified. Following the histopathological examination, immunohistochemistry was performed. Both tumors showed positive staining for estrogen receptor (ER) and progesterone receptor (PR) in tumor cells and WT1 negative, supporting the diagnosis of endometrioid carcinoma. P53 showed wild type pattern. Ki67 index had similar values across the different tumors, around 30-40% positive cells (Figure 2). When tested for microsatellite instability (MSI), the expression of PMS2 and MSH6 was kept in both tumors (Figure 3). Although the tumors showed histological and immunohistochemical similarities in both components, we did not find any continuity between the two tumors. This fact, together with the presence of associated lesions of endometriosis and adenomyosis, led to the final diagnosis of endometrioid SEOC with FIGO stage IA endometrial endometrioid adenocarcinoma and FIGO stage IIIc3 endometrioid ovarian carcinoma.
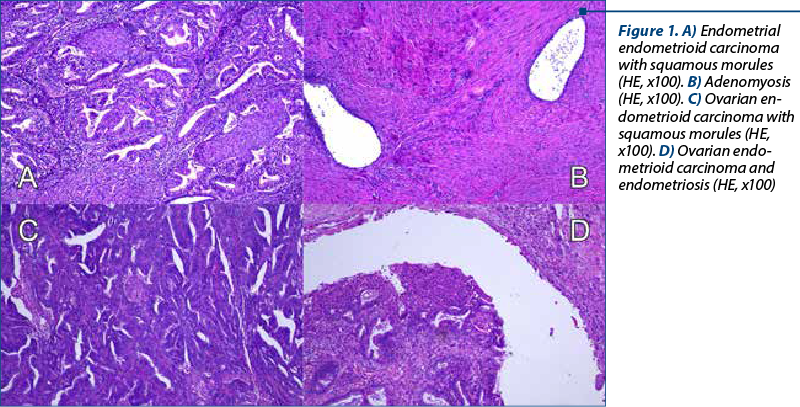
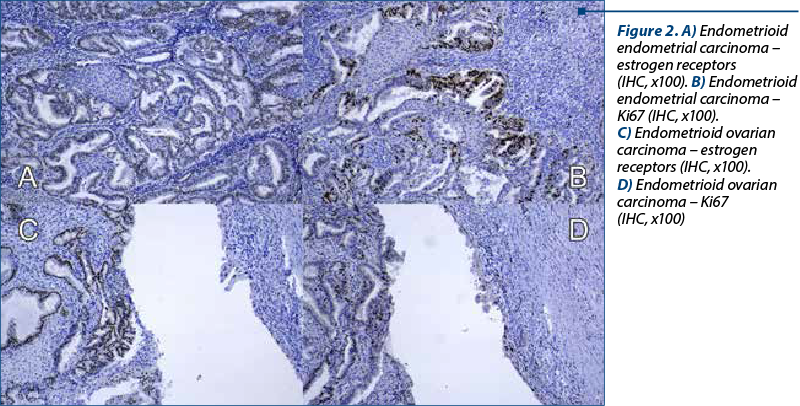
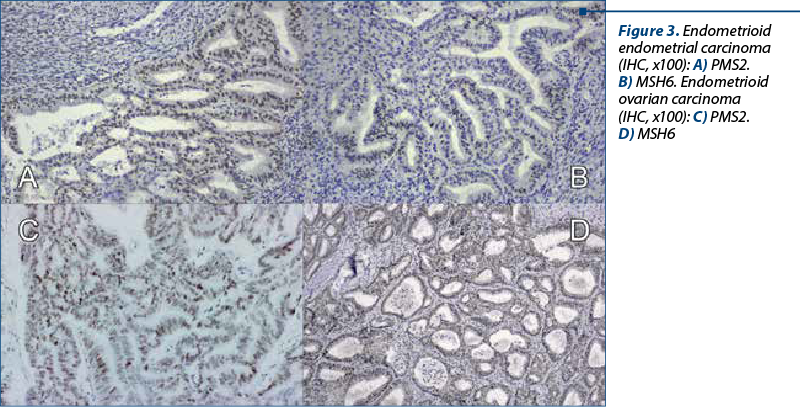
Discussion
Managing SEOC requires a multidisciplinary approach involving pathologists, gynecologists, oncologists, radiologists and other specialists (Figure 4). Tailored treatment strategies consider tumor stage, histological subtype, molecular profile, and patient’s general health status. Surgery, including total hysterectomy, bilateral salpingo-oophorectomy and lymph node dissection, is essential for early stages, while chemotherapy addresses advanced cases. Targeted therapies against specific molecular changes exhibit promise for improved outcomes.
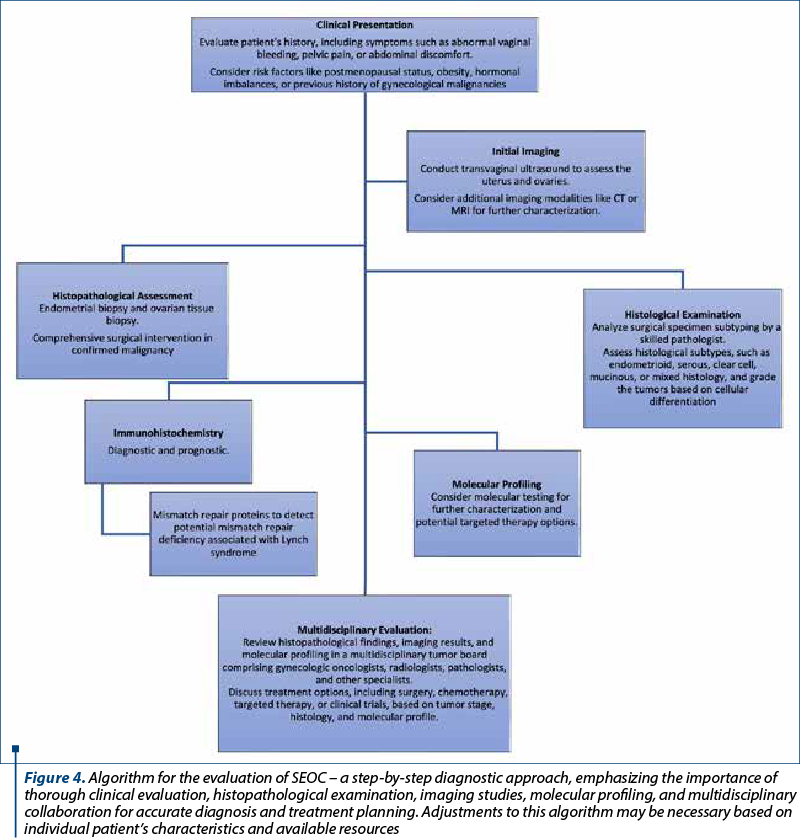
Prognostic factors for synchronous carcinomas encompass tumor stage, histology and molecular traits. Generally, the favorable prognosis aligns with endometrioid histology and early-stage disease versus high-grade, serous histology or advanced tumors. However, prognosis variability underscores the need for predictive biomarkers and innovative therapies to enhance outcomes.
Diagnosing, classifying and managing SEOC pose complex challenges. The accurate diagnosis of SEOC needs a thorough examination of histopathological characteristics together with supplementary investigations. Discriminating between primary endometrioid synchronous tumors and metastatic disease presents a significant challenge, demanding an extensive evaluation of morphology and sometimes immunohistochemistry. Moreover, distinguishing synchronous primaries from metastatic disease bears critical implications for both patient’s management and prognosis.
In cases of synchronous cancer, particularly involving SEOC, there might be an association with Lynch syndrome. Therefore, it is advisable to conduct testing for microsatellite instability (MSI) and perform immunohistochemistry to assess potential mismatch repair deficiencies(4,5). Lynch syndrome – also known as hereditary non-polyposis colorectal cancer (HNPCC) – is an inherited genetic condition linked to an increased risk of various cancers, including colorectal, endometrial, ovarian, and others.
Understanding their histopathology, molecular alterations and clinical impact is crucial. Collaborative efforts among experts and advancements in molecular profiling aid in development of treatment strategies. Further research into molecular mechanisms and novel therapeutic targets remains essential for advancing patient care and outcomes. SEOCs present a complex scenario in clinical settings, often raising debates about their origin, prognosis, and optimal management.
The presence of coexisting endometrial cancer potentially facilitates the earlier detection of ovarian cancers, notably observing a higher proportion of low-grade ovarian cancers in early stages, but this was not the case in our study(6). The significance of lymphovascular space invasion and infiltration into adjacent vasculature emerged as crucial prognostic factors in various malignancies, demonstrating predictive value for progression-free survival and disease-free survival(7). We did not identify lymphovascular invasion in our case.
The morphological criteria used for diagnosis include tumor grade and stage, as well as various features specific to each type of tumor. Studies have shown that low-stage and low-grade synchronous endometrioid carcinomas behave as independent primary tumors, often displaying similar survival outcomes to stage I endometrioid endometrial cancer without synchronous ovarian cancer(8). Several studies have explored ancillary methods, such as immunohistochemistry and molecular studies, to aid in the diagnostic process. While immunohistochemistry has shown some promise, especially with specific markers, its overall utility remains uncertain. Molecular studies, including microsatellite instability, X chromosome inactivation patterns, loss of heterozygosity and gene mutation analyses, have indicated clonal origins in most cases. Recent studies using next-generation sequencing have further supported the clonality of these tumors, highlighting shared mutations in specific genes among synchronous endometrioid endometrial and ovarian carcinomas(9,10).
However, the application of molecular analysis for differential diagnosis remains to be established, as some studies have suggested that molecular testing might not reliably differentiate between independent primary tumors and metastatic disease. Also, the molecular testing is not always available, especially due to costs.
Nevertheless, molecular studies have contributed to understanding the pathogenesis of these tumors and could have significance in clinical practice for prognosis and predictive purposes. Further studies involving more cases are essential to conclusively determine the role of molecular profiling in the diagnosis and management of these tumors. But until then, the diagnosis is in the hands of the multidisciplinary team that needs to correlate the entire spectrum of clinical and morphological data.
Conflict of interest: none declared.
financial support: none declared.
This work is permanently accessible online free of charge and published under the CC-BY licence.
Bibliografie
-
Makris GM, Manousopoulou G, Battista MJ, Salloum I, Chrelias G, Chrelias C. Synchronous endometrial and ovarian carcinoma: a case series. Case Rep Oncol. 2017;10(2):732-6.
-
Žilovič D, Čiurlienė R, Šidlovska E, Vaicekauskaitė I, Sabaliauskaitė R, Jarmalaitė S. Synchronous endometrial and ovarian cancer: A case report. World J Clin Cases. 2023;11(18):4341-9.
-
Rijal Y, Shrestha S, Bogati L, Regmi P, Shrestha S, Luitel P, Yadav CN, Khaniya B, Maskey S. Synchronous primary endometrium and ovarian carcinoma: A case report. Clin Case Rep. 2022;10(10):e6432.
-
Takeda T, Banno K, Yanokura M, Anko M, Kobayashi A, Sera A, Takahashi T, Adachi M, Kobayashi Y, Hayashi S, Nomura H, Hirasawa A, Tominaga E, Aoki D. Synchronous endometrial and ovarian cancer in Lynch syndrome with a MSH2 germline mutation: A case report. Mol Clin Oncol. 2018;9(5):479-84.
-
Fujii H, Matsumoto T, Yoshida M, et al. Genetics of synchronous uterine and ovarian endometrioid carcinoma: Combined analyses of loss of heterozygosity, PTEN mutation and microsatellite instability. Hum Pathol. 2002;33:421–8.
-
Khalid N, Ullah F, Zafar H, Anwer AW, Abbas T, Shakeel O, Faisal M, Sadaf T, Syed AA. Synchronous primary endometrial and ovarian cancers: trends and outcomes of the rare disease at a South Asian tertiary care cancer center. Cureus. 2020;12(7):e9163.
-
Anglesio MS, Wang YK, Maassen M, Horlings HM, Bashashati A, Senz J, Mackenzie R, Grewal DS, Li-Chang H, Karnezis AN, Sheffield BS, McConechy MK, Kommoss F, Taran FA, Staebler A, Shah SP, Wallwiener D, Brucker S, Gilks CB, Kommoss S, Huntsman DG. Synchronous endometrial and ovarian carcinomas: evidence of clonality. J Natl Cancer Inst. 2016;108(6):djv428.
-
Hoorshad N, Nassiri S, Najibi S, Feizabad E, Zamani N. Synchronous endometrial and ovarian cancer and its recurrent risk factors: Case series. Cancer Treat Res Commun. 2023;36:100731.
-
Hájková N, Tichá I, Hojný J, et al. Synchronous endometrioid endometrial and ovarian carcinomas are biologically related: A clinicopathological and molecular (next generation sequencing) study of 22 cases. Oncol Lett. 2019;17: 2207-14.
-
Kobayashi Y, Nakamura K, Nomura H, et al. Clinicopathologic analysis with immunohistochemistry for DNA mismatch repair protein expression in synchronous primary endometrial and ovarian cancers. Int J Gynecol Cancer. 2015;25:440–6.






