Systemic lupus erythematosus (SLE), a chronic autoimmune disease predominantly affecting women during their reproductive years, presents unique challenges during pregnancy. This retrospective study, conducted at the Obstetrics and Gynecology Department of the Emergency University Hospital Bucharest, between 2021 and 2023, analyzes three cases of SLE in pregnancy to elucidate maternal and neonatal outcomes. Through the analysis of three compelling cases, the study navigates the complexities of severe preeclampsia, SLE flares, thrombocytopenia, intrauterine growth restriction and preterm delivery, each demanding a nuanced approach. The findings underscore the critical role of interdisciplinary collaboration and meticulous medication management, with a specific focus on the compatibility and efficacy of hydroxychloroquine. By unraveling the diverse clinical presentations and outcomes, the study contributes with valuable insights into optimizing pregnancy care for women grappling with systemic lupus erythematosus.
Challenges and insights in managing systemic lupus erythematosus during pregnancy: a retrospective analysis of maternal and neonatal outcomes
Provocări şi perspective în managementul lupusului eritematos sistemic în timpul sarcinii: analiză retrospectivă a rezultatelor materne şi fetale
First published: 27 noiembrie 2023
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/Gine.42.4.2023.8984
Abstract
Rezumat
Lupusul eritematos sistemic (LES) este o boală cronică autoimună ce afectează predominant femeile aflate la vârsta fertilă şi care prezintă provocări unice în timpul sarcinii. Acest studiu retrospectiv a fost realizat în cadrul Departamentului de obstetrică-ginecologie al Spitalului Universitar de Urgenţă Bucureşti, pe o perioadă de trei ani, şi analizează trei cazuri de LES în sarcină, cu evidenţierea complicaţiilor impuse de această patologie: preeclampsie severă, puseuri de activitate a bolii, trombocitopenie, risc de naştere prematură şi restricţie de creştere intrauterină, fiecare necesitând o abordare nuanţată şi particularizată. Ţinând cont de riscurile impuse de această patologie, rolul unei echipe multidisciplinare este foarte important în stabilirea medicaţiei şi în managementul bolii, accentul fiind pus pe beneficiul administrării hidroxiclorochinei. Prin prezentarea celor trei cazuri clinice, studiul are contribuţii valoroase în optimizarea îngrijirii femeilor însărcinate cu lupus eritematos sistemic.
Introduction
Systemic lupus erythematosus (SLE) is a rare and chronic autoimmune disease with a broad spectrum of symptoms, including rash, arthritis, anemia, thrombocytopenia, serositis, nephritis, seizures and psychosis. It can range from mild to life-threatening, and it typically exhibits a fluctuating clinical course. Some patients may experience continuous disease activity(1,2). Systemic lupus erythematosus is marked by autoreactive T and B cells, leading to the generation of pathogenic autoantibodies and the deposition of immune complexes, resulting in tissue damage(3). The condition can affect various organ systems, such as the skin, musculoskeletal, pulmonary, cardiovascular, hematologic, renal and nervous systems. Diagnosing systemic lupus erythematosus poses challenges due to its gradual development and evolving course(4). Most SLE patients test positive for antinuclear antibodies (ANA) and often display other autoantibodies. The diagnosis necessitates the presence of ANA, other autoantibodies, and/or clinical manifestations(5). Addressing pregnancy in individuals with SLE is crucial due to the profound immunological and hormonal changes during this critical period, posing unique challenges and potential consequences for both the mother and the fetus.
Epidemiological studies have estimated the prevalence of systemic lupus erythematosus to be between 45.2 and 102.9 cases per 100,000, with an annual incidence of 2.4-7.2 cases per 100,000(6,7). This autoimmune disease primarily affects women, peaking during their reproductive years, with a female-to-male prevalence ratio ranging from 7:1 to 9:1. Genetic factors on the X chromosome, including Foxp3, TLR7, IRAK1 and CD40 ligand, alongside the influence of sex hormones contribute to immune dysregulation in women(8,9). The impact of pregnancy on SLE disease activity and vice versa is not unexpected, influencing pregnancy outcomes.
Although recent data suggest improvements in maternal mortality and fetal death rates, the incidence of caesarean section and preeclampsia remains higher in SLE pregnancies, showing limited changes over time. Careful management and interdisciplinary collaboration are crucial for optimizing outcomes in pregnancies complicated by systemic lupus erythematosus(10).
Managing disease activity and flares during pregnancy involves using medications that are effective yet safe for the developing fetus. Concerns about potential toxicity often lead patients, and sometimes even physicians, to discontinue medications, resulting in avoidable disease flares and associated consequences. Limited data on drug safety during pregnancy are typically derived from registries, case reports or animal studies. Despite these challenges, there are multiple effective options, and the choices should be made by weighing maternal benefit against fetal toxicity(11,12).
The purpose of this article is to investigate and present the clinical details, progression and management of systemic lupus erythematosus during pregnancy. Through the analysis of three distinct cases, we aim to highlight specific challenges such as severe preeclampsia, SLE flares and thrombocytopenia, providing insights into effective therapeutic approaches.
Materials and method
This article is a retrospective study that took place between 2022 and 2023 in the Obstetrics and Gynecology Department of the Emergency University Hospital Bucharest, where three cases of systemic lupus erythematosus in pregnancy were chosen, and we present here the maternal and neonatal outcomes. The patients’ history was gathered by reviewing the hospital records, encompassing demographic information, current pregnancy details, maternal outcomes, fetal outcomes, laboratory investigations, and the SLE-related treatment provided.
Case series
A series of three cases of SLE in pregnancy are presented.
Case 1
A 28-year-old primigravida with 34 weeks of gestation, known with systemic lupus erythematosus since the age of 14, with antiphospholipid syndrome (APS), positive anti-Ro and anti-La antibodies and hypertension, presented to the emergency room with severe headache.
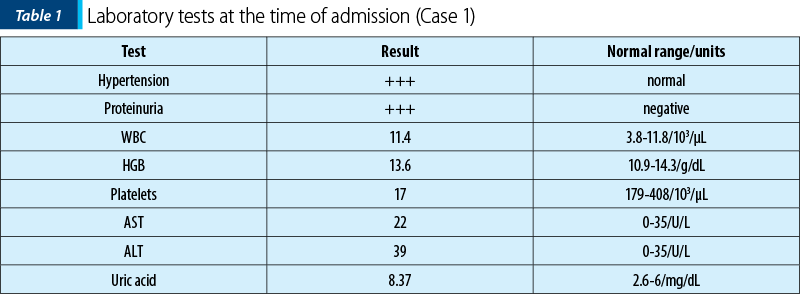
From the patient’s personal medical history, she was diagnosed with systemic lupus erythematosus at the age of 14, experiencing multiple vascular, cutaneous, muscular, hematological, hepatic, serosal complications, lupus endocarditis, lupus nephropathy, and multiple ischemic strokes in the carotid territory. Prior to pregnancy, the patient underwent treatment with cyclophosphamide, corticosteroid therapy and acenocoumarol. Upon confirmation of pregnancy, acenocoumarol was discontinued, and the patient began the daily treatment with enoxaparin sodium 0.4 ml, acetylsalicylic acid 75 mg, hydroxychloroquine 200 mg, methyldopa 250 mg, and nifedipine 40 mg. During pregnancy, the patient was lost to follow-up regarding rheumatology condition. At the time of admission, she was in a hypertensive crisis, with a systolic blood pressure of 230 mmHg. Laboratory analyses indicated significant proteinuria, normal leukocyte count, severe thrombocytopenia, elevated uric acid, normal hemoglobin levels, and a slight increase in transaminases (Table 1). A pregnancy ultrasound, along with cardiology, internal medicine, nephrology and hematology consultations were conducted. Immediate hospitalization led to the decision to perform a caesarean section, with intraoperative platelet transfusion, due to severe preeclampsia and unresponsive hypertensive crisis to medical therapy. A live female infant, weighing 1910 g, with an Apgar score of 8, was delivered through the procedure, extracting the baby from the cranial presentation. After the neonatal assessment, the findings revealed intrauterine growth restriction (IUGR) and right bundle branch block which will require subsequent cardiac monitoring. Inflammatory biomarkers evaluated dynamically were consistently negative throughout the hospitalization.
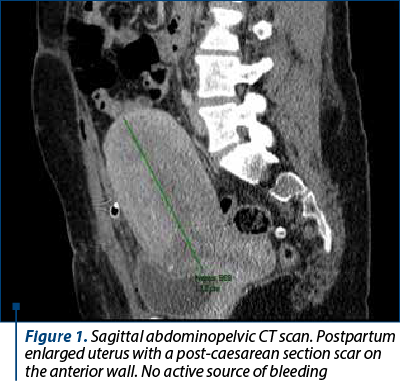
Postoperatively, there was a rapid progressive decrease in hemoglobin levels to severe anemia (6.1 g/dL), with platelet levels remaining stable, with a slight tendency to decrease. The decision was made to proceed with blood transfusion, platelet transfusion and an abdominopelvic CT scan, which revealed no active sources of bleeding (Figure 1). A direct Coombs test was performed, and the result was negative.
Together with the rheumatology team, based on the dynamic laboratory values and the symptomatology, it was concluded that the patient was in a SLE flare. The decision was made to administer methylprednisolone 1 g/day in pulse therapy for three days.
The patient’s condition improved favorably, with the correction of pancytopenia and a decrease in blood pressure values. She was discharged after eight days of hospitalization.
Case 2
A 36-year-old primiparous with 33 weeks of gestation, known with systemic lupus erythematosus, immune thrombocytopenic purpura and positive anti-Ro antibodies, presented to the emergency room with uterine contractions and minimal metrorrhagia.
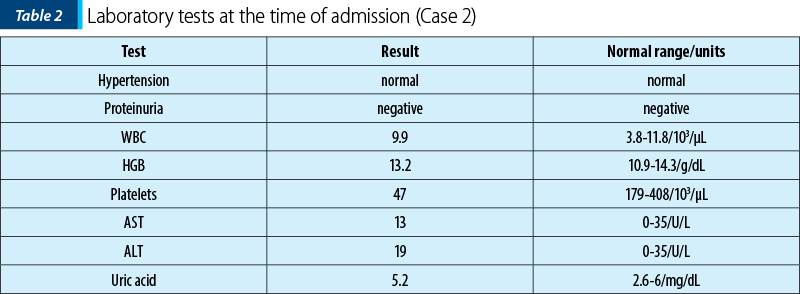
The laboratory analyses indicated normal leukocyte count, severe thrombocytopenia, normal uric acid and normal hemoglobin levels (Table 2).
During pregnancy, the patient attended regular visits to the rheumatologist and was undergoing treatment with hydroxychloroquine 200 mg/day and prednisone 20 mg/day. Tocolytic treatment was initiated with nifedipine 40 mg/day and progesterone to prevent premature birth. Platelet transfusion was administered to correct thrombocytopenia. On the second day of hospitalization, a decision was made and a caesarean section was performed, resulting in the birth of a male infant weighing 1950 g, with an Apgar score of 7 at one minute and 8 at five minutes, with a double nuchal cord. The newborn had a normal weight for gestational age, mild respiratory distress syndrome, grade I intracranial hemorrhage, normal platelet count and inflammatory markers within normal ranges.
Postoperatively, an additional unit of platelet concentrate was administered, along with hematological consultations. The treatment was continued, and an additional dose of dexamethasone (16 mg/day) was administered.
The patient’s progress was favorable, with a hospitalization duration of 15 days, with no occurrence of a lupus flare, and correction of thrombocytopenia (at discharge, the platelets were 127/103).
Case 3
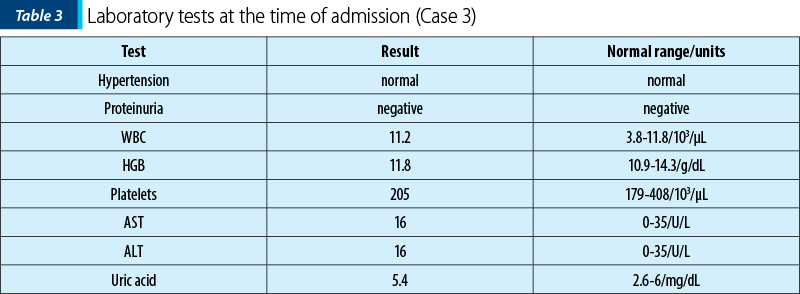
A 38-year-old primiparous patient at 39 weeks of pregnancy, known to have systemic lupus erythematosus, presented to the emergency room with painful uterine contractions. At the time of admission, the patient showed no significant changes in laboratory analyses, with normal uric acid and no proteinuria (Table 1). She had not experienced an active disease flare for six months preconception and during pregnancy, and she attended regular visits to the rheumatologist. The medication during pregnancy included hydroxychloroquine 200 mg per day and prednisone 10 mg per day.
The patient gave birth through a caesarean section to a full-term male infant weighing 3900 g, with an Apgar score of 10. After the neonatal examination, it was observed that the newborn was large for gestational age (LGA). Additionally, there was noticed hypoplasia of the triangular nerve of the oral commissure, requiring subsequent neurological follow-up. The platelet count was normal, with only a higher level of C-reactive protein recorded, but it normalized within three days.
The postoperative course was favorable, without SLE flare and without any other complications, and she was discharged after four days of hospitalization.
Discussion
Women with SLE anticipating pregnancy should be tested for the presence of anti-Ro/SSA, anti-La/SSB and antiphospholipid antibodies – e.g., anticardiolipin IgG and IgM, b2-glycoprotein-I IgG and IgM, and the lupus anticoagulant (LAC); these antibodies portend serious complications during pregnancy. Moderate and high titers of antiphospholipid antibodies in combination with the LAC confer the highest risk for poor fetal and maternal outcomes(13,14). In our study, the first patient was known to have antiphospholipid syndrome and present anti-Ro and anti-La antibodies. Her progression showed multiple complications throughout the pregnancy. In the second case presented, the patient had anti-Ro antibodies present during pregnancy, with only the occurrence of thrombocytopenia and premature birth.
Various research findings indicate a connection between an active disease at conception and the likelihood of experiencing disease flares throughout pregnancy. A comprehensive study encompassing 55 pregnancies among 39 women diagnosed with systemic lupus erythematosus systematically collected clinical and laboratory data from six months before pregnancy to the first year post-partum. The study revealed that a history of nephritis and elevated disease activity, assessed through the SLE Disease Activity Index (SLEDAI), were predictive factors for adverse maternal outcomes during pregnancy(15). In the prospective study “Predictors of Pregnancy Outcome: Biomarkers in Antiphospholipid Antibody Syndrome and Systemic Lupus Erythematosus” (PROMISSE), less than 10% of 318 patients with mild to moderate disease during the initial trimester of pregnancy encountered mild flares, with only 3% experiencing severe flares(16). In our study, the first patient experienced multiple flare episodes, including four months before becoming pregnant, with multiple organ involvements such as lupus nephritis, lupus endocarditis, and a history of multiple ischemic strokes. Immediately postnatally, she exhibited a lupus flare, accompanied by the onset of pancytopenia within a two-day interval.
Distinguishing between a systemic lupus erythematosus flare and preeclampsia is challenging, especially since preeclampsia complicates up to 20% of lupus pregnancies. This distinction is crucial because preeclampsia requires immediate delivery, while SLE flare is managed with immunosuppression. Both conditions may present with hypertension and proteinuria, but urinalysis in preeclampsia is less likely to show an active sediment than in SLE flare. Thrombocytopenia is common to both, but elevated liver function tests lean more towards preeclampsia. In lupus flares, lower white blood cell counts, complement levels and uric acid are observed compared to preeclampsia(17). In the first case presented, laboratory analyses strongly indicated severe preeclampsia, with significant proteinuria, hypertension, severe thrombocytopenia, normal white blood cell count, elevated uric acid levels, and mild alterations in transaminase levels, all present from the time of admission.
A study from Taiwan, involving 2059 offspring of individuals with systemic lupus erythematosus, revealed elevated rates of intrauterine growth restriction, preterm birth and stillbirth(18). Similarly, an Italian cohort reported an increased risk of preterm delivery and infants classified as small for gestational age(19). In pregnancies complicated by systemic lupus erythematosus, adverse outcomes are more prevalent compared to pregnancies in the general population. A study examining 4000 SLE pregnancies revealed longer hospital stays, increased hypertension, higher rates of intrauterine growth restriction (IUGR), and an elevated likelihood of caesarean sections(20). This increased caesarean section rate is influenced by both medical necessity and patient/provider preferences. Of the three cases presented in our study, two of them gave birth prematurely at 34 and 33 weeks, respectively; continuing the pregnancy posed a risk to both maternal and fetal lives. All patients included in our study gave birth through a caesarean section.
Among the three cases presented in our study, two newborns were premature, one exhibited intrauterine growth restriction, and one was large for the gestational age. The literature reported increased occurrences of intrauterine growth restriction, premature delivery and fetal demise(18). Anti-Ro/SSA and anti-La/SSB antibodies are found in about one-third of women with SLE. Neonatal lupus (NL) can manifest in 10% of infants born to mothers with these antibodies, showcasing cutaneous or cardiac symptoms. Congenital complete heart block (CCHB) occurs in 1-2% of fetuses from mothers with anti-Ro and anti-La antibodies, and this risk rises to 17% if the mother has a prior history of a child with CCHB. In the first case presented, the newborn had a form of neonatal lupus with cardiac manifestations, specifically right bundle branch block. The mother was tested positive for anti-Ro and anti-La antibodies, a finding consistent with the literature.
The management of medication during pregnancy must carefully consider the potential risks to the developing fetus. Hydroxychloroquine is deemed compatible with pregnancy(21). Notably, studies of neonates exposed to hydroxychloroquine in utero have not indicated retinal toxicity or prolonged neonatal QTc intervals(22). Multiple studies have shown that maintaining hydroxychloroquine during pregnancy decreases disease flares and lowers the risk of preeclampsia. Furthermore, this medication is considered compatible with breastfeeding(21). Steroids can be continued during pregnancy for optimal disease control, but efforts should be made to minimize exposure. High doses are associated with an increased risk of complications, including diabetes, hypertension, preeclampsia and premature rupture of membranes. However, short-term use for flares and disease control is permissible(23). Immunosuppressive agents, like cyclophosphamide, methotrexate and mycophenolate, have teratogenic potential and should ideally be discontinued before conception. Safe immunosuppressants for pregnancy use include azathioprine and the calcineurin inhibitors tacrolimus and cyclosporine, as numerous studies have demonstrated their safety and efficacy during pregnancy(12,23). All patients included in our study underwent treatment with hydroxychloroquine, prednisone and dexamethasone. Immunosuppressive agents with teratogenic potential were discontinued at the time of conception.
Conclusions
Elevated lupus activity is observed in the presence of an excess of hormones and various physiological changes during pregnancy, making it challenging to differentiate between lupus activity and normal pregnancy features. The heightened inflammatory response during a lupus flare poses significant risks for both maternal and fetal well-being. It is crucial to emphasize the contraception use, especially when patients are on teratogenic medications. Effectively managing systemic lupus erythematosus during pregnancy necessitates a coordinated approach involving the patient, the obstetrician, the rheumatologist, the nephrologist, the hematologist, the cardiologist and the neonatologist.
Conflict of interest: none declared.
financial support: none declared.
This work is permanently accessible online free of charge and published under the CC-BY licence.

Bibliografie
-
Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358(9):929–39.
-
Rees F, Doherty M, Grainge M, Davenport G, Lanyon P, Zhang W. The incidence and prevalence of systemic lupus erythematosus in the UK, 1999-2012. Ann Rheum Dis. 2016;75(1):136–41.
-
Choi J, Kim ST, Craft J. The pathogenesis of systemic lupus erythematosus – an update. Curr Opin Immunol. 2012;24(6):651–7.
-
Bertsias GK, Pamfil C, Fanouriakis A, Boumpas DT. Diagnostic criteria for systemic lupus erythematosus: has the time come? Nat Rev Rheumatol. 2013;9(11):687–94.
-
Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology. Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheumatol. 2019;71(9):1400–12.
-
Stojan G, Petri M. Epidemiology of systemic lupus erythematosus: an update. Curr Opin Rheumatol. 2018;30(2):144–50.
-
Gergianaki I, Bortoluzzi A, Bertsias G. Update on the epidemiology, risk factors, and disease outcomes of systemic lupus erythematosus. Best Pract Res Clin Rheumatol. 2018;32(2):188–205.
-
Tedeschi SK, Bermas B, Costenbader KH. Sexual disparities in the incidence and course of SLE and RA. Clin Immunol. 2013;149(2):211–8.
-
Gaudreau MC, Johnson BM, Gudi R, Al-Gadban MM, Vasu C. Gender bias in lupus: does immune response initiated in the gut mucosa have a role? Clin Exp Immunol. 2015;180(3):393–407.
-
Mehta B, Luo Y, Xu J, et al. Trends in maternal and fetal outcomes among pregnant women with systemic lupus erythematosus in the United States: a cross-sectional analysis. Ann Intern Med. 2019;171(3):164–71.
-
Lateef A, Petri M. Management of pregnancy in systemic lupus erythematosus. Nat Rev Rheumatol. 2012;8(12):710–8.
-
Götestam Skorpen C, Hoeltzenbein M, Tincani A, Fischer-Betz R, Elefant E, Chambers C, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis. 2016;75(5):795–810.
-
Allen D, Hunter MS, Wood S, Beeson T. One Key Question®: First Things First in Reproductive Health. Matern Child Health J. 2017;21(3):387–92.
-
Yelnik CM, Porter TF, Branch DW, et al. Brief report: changes in antiphospholipid antibody titers during pregnancy: effects on pregnancy outcomes. Arthritis Rheumatol. 2016;68(8):1964–9.
-
Kwok LW, Tam LS, Zhu T, Leung YY, Li E. Predictors of maternal and fetal outcomes in pregnancies of patients with systemic lupus erythematosus. Lupus. 2011;20(8):829–36.
-
Buyon JP, Kim MY, Guerra MM, et al. Predictors of pregnancy outcomes in patients with lupus: a cohort study. Ann Intern Med. 2015;163(3):153–63.
-
Bellos I, Pergialiotis V, Loutradis D, Daskalakis G. The prognostic role of serum uric acid levels in preeclampsia: A meta-analysis. J Clin Hypertens Greenwich Conn. 2020;22(5):826–34.
-
Chen YJ, Chang JC, Lai EL, et al. Maternal and perinatal outcomes of pregnancies in systemic lupus erythematosus: A nationwide population-based study. Semin Arthritis Rheum. 2020;50(3):451–7.
-
Moroni G, Doria A, Giglio E, et al. Fetal outcome and recommendations of pregnancies in lupus nephritis in the 21st century. A prospective multicenter study. J Autoimmun. 2016;74:6–12.
-
Chakravarty EF, Nelson L, Krishnan E. Obstetric hospitalizations in the United States for women with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum. 2006;54(3):899–907.
-
Davidov D, Sheiner E, Wainstock T, Miodownik S, Pariente G. Maternal Systemic Lupus Erythematosus (SLE). High risk for preterm delivery and not for long-term neurological morbidity of the offspring. J Clin Med. 2021;10(13):2952.
-
Friedman DM, Kim M, Costedoat-Chalumeau N, et al. Electrocardiographic QT intervals in infants exposed to hydroxychloroquine throughout gestation. Circ Arrhythm Electrophysiol. 2020;13(10):e008686.
-
Østensen M, Khamashta M, Lockshin M, et al. Anti-inflammatory and immunosuppressive drugs and reproduction. Arthritis Res Ther. 2006;8(3):209.
Articole din ediţiile anterioare
Managementul sarcinii asociate cu transplant renal şi infecţie cu SARS-CoV-2 – prezentare de caz
Sarcinile asociate transplantului renal implică deseori complicaţii materno-fetale. Riscul de preeclampsie, moarte fetală intrauterină sau de o...
Borelioza în sarcină - prezentare de caz şi review al literaturii
Borelioza reprezintă una dintre cele mai frecvente boli transmise de căpuşe. Diagnosticul clinic este pus de multe ori cu dificultate, din cauza mu...
Managementul ante-, peri- şi postnatal al unei gravide cu histiocitoză pulmonară cu celule Langerhans - prezentare de caz
Histiocitozele cu celule Langerhans sunt un grup de patologii rare produse prin proliferarea clonală a celulelor dendritice Langerhans, care pot af...
Advancements in preeclampsia: innovative screening methods and effective prevention strategies
Preeclampsia is a condition that affects 5-10% of pregnancies, being considered a systemic vascular dysfunction that is characterized by proteinuri...