Managementul sarcinii asociate cu transplant renal şi infecţie cu SARS-CoV-2 – prezentare de caz
Therapeutic strategy of pregnancy associated with renal transplant and SARS-CoV-2 infection – case report
Abstract
Pregnancy in women with renal transplant is associated with both maternal and fetal adverse events. The rates of preeclampsia, still birth or caesarean section are significantly higher than those in the general population. In these cases, the SARS-CoV-2 infection leads to an increased mortality rate. The management of pregnancy-associated renal transplant requires a multidisciplinary approach.Keywords
pregnancykidney transplantmanagementSARS-CoV-2 infectionRezumat
Sarcinile asociate transplantului renal implică deseori complicaţii materno-fetale. Riscul de preeclampsie, moarte fetală intrauterină sau de operaţie cezariană este semnificativ crescut faţă de cel din populaţia generală. În aceste cazuri, supraadăugarea infecţiei cu SARS-CoV-2 duce la creşterea mortalităţii materno-fetale. Managementul sarcinilor asociate cu transplant renal necesită o abordare multidisciplinară.Cuvinte Cheie
sarcinătransplant renalmanagementinfecţie cu SARS-CoV-2Introduction
Chronic kidney disease, widely classified into five stages according to the level of renal function, is often clinically silent until renal impairment is advanced(1). Although the symptoms become obvious when the glomerular filtration rate declines to less than 25% of normal, half of the renal function may be damaged before serum creatinine rises above 1.35 mg/dl(2-4).
Stages 3-5, which complicate one in 750 pregnancies, are characterized by a glomerular filtration rate under 60 ml/min, involving reduced fertility and having a high risk of early miscarriages(1,4).
Women with end-stage renal disease have impaired fertility due to the disruption of hypothalamic gonadal axis. Since there is a rapid restoration of fertility, kidney transplantation offers the best hope to women with end-stage renal disease who wish to become pregnant(5-7).
A case-control study of 360 women with mild renal dysfunction (serum creatinine less than 1.24 mg/dl), minimal proteinuria (<1 g/24 h), and absent or well controlled hypertension before pregnancy showed that pregnancy had no adverse effect on renal function(1,8).
On the other hand, patients with moderate or severe renal impairment had a decline in renal function during pregnancy, especially in cases associated with preexisting proteinuria and hypertension(9).
A study performed on 87 pregnancies with moderate renal disease showed a risk of 40% of a decline in renal function during pregnancy, which persisted after birth in half of the cases. Approximately 65% of the patients with severe disease had a decline in renal function during the first trimester, and 28% deteriorated to end-stage renal failure(1,4).
Pregnancy in a kidney transplant recipient continues to remain challenging due to the risk of adverse maternal complications of preeclampsia and hypertension, and the risk of adverse fetal outcomes of premature birth, low birth weight, and small-for-gestational-age infants(6). Additionally, there is risk of side effects from immunosuppressive medication, and the risk of deterioration of the allograft function(5,10). Therefore, preconception counseling, family planning and contraception are pertinent parts of the transplant counseling process(10).
Case report
A 31-year-old woman, secundigravida, known with right kidney transplant, was referred to the Bucharest University Emergency Hospital, presenting an altered condition and painful uterine contractions. She had a 37-38-week ongoing pregnancy and she was recently diagnosed with SARS-CoV-2 infection, after presenting dry cough and sore throat.
Her pregnancy medical history revealed hereditary thrombophilia (Factor V Leiden’s and MTHFR’s mutations), antiphospholipid syndrome and a treated genital infection with Streptococcus group B. Also, the ultrasound examinations revealed an intrauterine growth restriction, which appeared in the 34th week of gestation.
Her renal medical history revealed urinary tract infections in childhood, therapeutically neglected and subsequently without significant personal pathological history known/medically documented until seven years ago, when a routine examination for pregnancy detected nitrogen retention syndrome (creatinine 7.32 mg/dl, urea 133.2 mg/dl) and secondary anemia (Hb 8.4 g/dl). The pregnancy was interrupted on request at 8 weeks.
She was admitted in the Renal Transplant Clinic of the Fundeni Clinical Institute in 2015, with the diagnosis of stage V chronic kidney disease, chronic pyelonephritis, secondary renal anemia, secondary hyperparathyroidism and secondary renal parenchymal hypertension. The kidney transplant was performed in November 2015, from a donor in brain death, with Simulect induction. No surgical, immunological or infectious complications after kidney transplantation were noted.
At the admission to our hospital, the patient was hemodynamically stable, conscious, with a systolic blood pressure of 160 mmHg and SaO2=98% in atmospheric air.
The patient was evaluated in a multidisciplinary team (obstetrician, nephrologist, cardiologist, specialist in infectious disease) and she was admitted in the obstetrics and gynecology department for fetal evaluation.
The laboratory findings showed a mild anemia (Hb 8.7 g/dl), lymphopenia (16.4%), thrombocytopenia (154.000/mm3), a slightly increased renal function (creatinine 1.53 mg/dl, urea 61.4 mg/dl), elevated uric acid (12.15 mg/dl), hypertriglyceridemia (511 mg/dl) and hypomagnesaemia (1.56 mg/dl). The urine summary revealed a slight proteinuria (30 mg/dl). All the cultures (urine, cervix) were negative. The thrombophilia profile revealed a decreased value of protein S (25%).
The patient was undergoing immunosuppressive (tacrolimus 20 mg, azathioprine 100 mg, prednisone 5 mg), anticoagulant (enoxaparin 0.4 ml in the morning and 0.6 ml in the evening), and antihypertensive (methyldopa 250 mg twice a day) therapy.
Renal ultrasound revealed the kidney transplant measuring 120/70 mmHg, with pyelocaliceal dilation and an increased diameter of the ureter (1 cm). The nephrological examination revealed a decreased glomerular filtration rate (50 ml/min/1.73 m2) and a diuresis of 1800 ml/24 h.
The cardiology examination diagnosed the preexisting hypertension of pregnancy, without the appearance of ventricular hypertrophy on the ECG and indicated the administration of one tablet of methyldopa at each 6 hours.
The specialist in infectious diseases decided to introduce remdesivir therapy due the SARS-CoV-2 infection, and antibiotics (ceftriaxone).
During the hospitalization, her blood pressure values were uncontrolled under maximum treatment, therefore, given that and the maternal pathology (kidney transplant and SARS-CoV-2 infection), a caesarean section was required. A 2600 g newborn, with mild signs of intrauterine growth restriction, was extracted. The laboratory findings of the newborn showed 0.02 IgG SARS-CoV-2 and 0.20 IgM SARS-CoV-2.
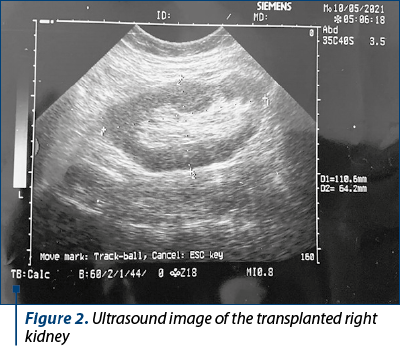
Intraoperatively, an extraperitoneal right kidney was visualized, positioned according to transplant standards, at the iliac artery origin, with minimal mobility; the ureter’s path was inspected through the transparency of the peritoneal leaf; also, unlike the rectilinear ultrasound path, it was characterized by a sinuous one, with preserved peristalsis. The uretero-hydronephrosis described by ultrasound was improved after surgery, as the images below show (Figure 2).
Postoperatively, the patient had a good surgical outcome, with a persisting cough within the respiratory infection. After 24 hours, according to the indications of the specialist in infectious diseases, the immunosuppressive treatment was modified due to the worsened respiratory symptoms (azathioprine was stopped and after dosing the tacrolimus’s serum values, the dose was halved).
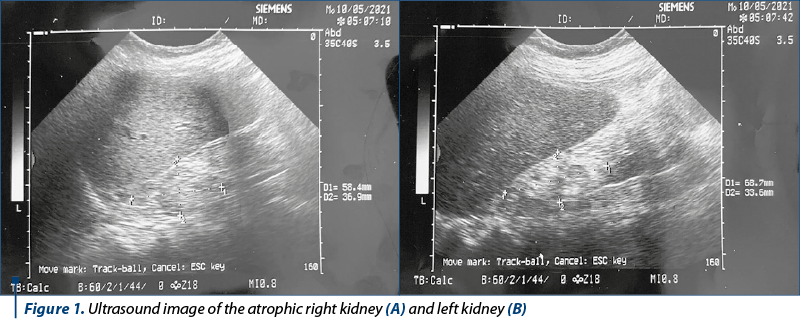
Renal ultrasound performed after caesarean section detected the left kidney measuring 58/36 mm, and the right kidney measuring 68/35 mm, both with irregular contour and with erasure of the cortico-medullary differentiation, without pyelic dilation. The kidney transplant, measuring 110/64 mm, had a regular contour, with normal parenchymal index and without pyelocaliceal dilation (Figures 1 and 2).
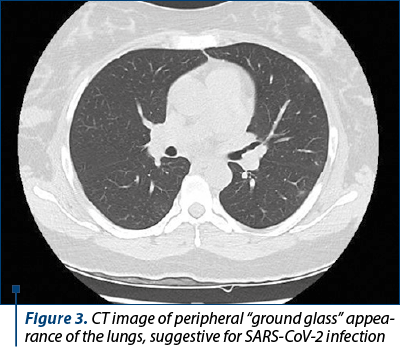
A computed tomography was performed after caesarean section; it detected disparate pseudo-nodular or macronodular foci of pulmonary condensation, with the aspect in “ground glass”, located predominantly peripheral or subpleural, in the upper and lower lobes, without pleural fluid (Figure 3). The laboratory findings in dynamics showed an improvement of the renal function, with decreasing values of urea (45 mg/dl) and a stationary value of creatinine (around 1.3 mg/dl). The secondary renal anemia was moderate after surgery (Hb 8.4 g/dl). Ferritin’s values were increased due to the coronavirus infection (from 348 ng/ml to 582 ng/ml). The reactive protein C decreased from 3.3 to 1.05 mg/dl under treatment. We observed an increase of the values of urea six days after surgery (86 mg/dl).
The patient was discharged after nine days of hospitalization, with improved respiratory symptoms and stationary renal function.
Discussion
Frequently, chronic kidney disease during pregnancy includes primary glomerulonephritis (including hypertension, proteinuria or recurrent infection), polycystic kidney disease (impaired renal function, hypertension), congenital urinary tract obstruction, vesicoureteral reflux nephropathy, nephrolithiasis, diabetes nephropathy and lupus nephritis(1,11). In our case, the underlying pathology that led to chronic kidney failure and the need for kidney transplantation was untreated urinary tract infections which led to chronic pyelonephritis. Although the glomerular filtration rate reached 7.1 ml/min/1.73 m2, according to CKD-EPI formula, the accidental discovery of renal failure was caused by the absence of symptoms, contradicting the definition according to which the symptoms appear when the glomerular filtration rated reduces to less than 25% of normal(1,8).
Chronic stage V kidney disease is associated with infertility, through impaired hypothalamic function, causing high levels of FSH, LH and prolactin, vasomotor dysfunction, prescribed medications and psychological factors. Although the pregnancy rate varies between 0.9% and 7%, our patient obtained spontaneously two pregnancies(12-14).
Kidney transplantation improves fertility rate a few months postoperatively (six months), leading to the reversal of hormonal dysfunction translated by normal ovulatory cycles and regular menstruation(12,13,15,16).
Obtaining a pregnancy is indicated two years after a kidney transplant, with the following conditions: serum creatinine less than 1.5 mg/dl, proteinuria less than 500 mg/24 h, without infections, without teratogenic medication, and stable immunosuppressive treatment(12,17). All these conditions were met in our case, mentioning that the pregnancy was obtained after five years and the medium value of serum creatinine was during this period around 1.35 mg/dl. Also, the associated hyperuricemia is frequent in renal transplant recipients.
Maternal kidney transplantation in associated with multiple maternal-fetal complications. Preeclampsia, the most common complication, can lead to kidney failure, HELLP syndrome, seizures, liver failure, stroke and maternal death. The diagnosis of preeclampsia may be difficult in patients with renal transplant due to the late onset of hypertension and to preexisting proteinuria(5,18). In our case, the hypertension preexisted the pregnancy and proteinuria was minimal. We must mention that the hypertension was stable before 36 weeks of gestation, under two tablets of methyldopa. While 20% of pregnant women develop early preeclampsia, in our case the blood pressure values became unstable under treatment late in pregnancy and the proteinuria was insignificantly increased.
Due to the low medium value of serum creatinine during pregnancy (below 1.4 mg/dl)(1,19), we can assume that the satisfactory adaptation in the gestational increase in renal blood flow was decisive for the favorable prognosis of the pregnancy. In accordance with a study assessing the decline of renal function which showed an accelerated decline during pregnancy in patients with an estimated glomerular filtration rate below 40 ml/min/1.73 m2, in our case the renal function was relatively stationary during the pregnancy, with an estimated glomerular filtration rate of 50 ml/min/1.73 m2 and a slight increase in serum urea values during the third trimester (from a medium of 45 mg/dl to 60 mg/dl). Although renal transplant is associated with recurrent urinary tract infections, in our case all the urinalyses performed in pregnancy were negative(6,25). Also, renal graft dysfunction during pregnancy and the rejection of the graft are frequent complications in pregnancy. Neither one appeared in our case, but the patient must be evaluated systematically due to the high risk of graft loss in the first two years postpartum(6,25).
In general, fetal complications involve fetal growth restriction, hypoxic neurological damage, fetal intrauterine death, and low birth weight. In our case, the fetal growth restriction had a late onset, at 34 weeks of pregnancy, and the fetal prognosis was favorable.
According to a brief review of COVID-19 in renal transplant recipients, the presentations of SARS-CoV-2 infected renal transplant patients were not different from the ones of the general population. Blood results showed that all patients had lymphopenia and high CRP and 35% had elevated serum ferritin, as in our case. SARS-CoV-2 infection increases the risk of preterm birth, intrauterine fetal growth and stillbirth(20,21). The laboratory findings of SARS-CoV-2 infection are, in our case, lymphopenia, thrombocytopenia, an increased level of reactive protein C; also, the abnormalities in thorax computed tomography are suggestive. Because the response of the immune system at viral infection can cause severe symptoms in an already immunosuppressed pregnancy woman, the intensive treatment of COVID-19 was required.
Another risk factor in this case was represented by the presence of antiphospholipid syndrome, which associated renal complications such as nephropathy, renal artery stenosis and end-stage kidney disease(22). The state of hypercoagulability established by thrombophilia and antiphospholipid syndrome is increased by SARS-CoV-2 infection, therefore the anticoagulant treatment was absolutely necessary(23,24).
The immunosuppressive treatment may be assessed in terms of correlation with the negative outcome of pregnancies. In our case, the renal function after transplantation was stable under tacrolimus and azathioprine therapy, so there was no change in the treatment schedule, although it is known to be associated with preterm and low birth weight(26,27).
Conclusions
The prognostic of pregnancy-associated renal transplant is burdened by the appearance of both maternal and fetal adverse outcomes, including increased rates of preeclampsia, gestational diabetes, caesarean section, prematurity and low birth rate. In addition, the SARS-CoV-2 infection determines a higher risk of preterm births.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
- Williams D, Davison J. Chronic kidney disease in pregnancy. BMJ. 2008;336 (7637):211-5.
- Fischer MJ, Lehnerz SD, Hebert JR, Parikh CR. Kidney disease is an independent risk factor for adverse fetal and maternal outcomes in pregnancy. Am J Kidney Dis. 2004;43(3):415-23.
- Imbasciati E, Gregorinin G, Cabiddu G, Gammaro L, Ambroso G, Del Giudice A, et al. Pregnancy in CKD stages 3 to 5: fetal and maternal outcomes. Am J Kidney Dis. 2007;49(6):753-62.
- Jones DC, Hayslett JP. Outcome of pregnancy in women with moderate or severe renal insufficiency. N Engl J Med. 1996;335(4):226-32.
- Shah S, Venkatesan RL, Gupta A, Sanghavi MK, Welge J, Johansen R, Verma P. Pregnancy outcomes in women with kidney transplant: Metaanalysis and systematic review. BMC Nephrology. 2019;20(1):1-22.
- Shah S, Verma P. Overview of pregnancy in renal transplant patients. Int J Nephrol. 2016;2016:4539342.
- Saha MT, Saha HH, Niskanen LK, Salmela KT, Pasternack AI. Time course of serum prolactin and sex hormones following successful renal transplantation. Nephron. 2002;92(3):735–7.
- Jungers P, Houillier P, Forget D, Labrunie M, Skhiri H, Giatras I, et al. Influence of pregnancy on the course of primary chronic glomerulonephritis. Lancet. 1995;346(8983):1122-4.
- Fitzpatrick A, Mohammadi F, Jesudason S. Managing pregnancy in chronic kidney disease: improving outcomes for mother and baby. Int J Womens Health. 2016;8:273-85.
- Sifontis NM, Coscia LA, Constantinescu S, Lavelanet AF, Moritz MJ, Armenti VT. Pregnancy outcomes in solid organ transplant recipients with exposure to mycophenolate mofetil or sirolimus. Transplantation. 2006:82(12):1698-702.
- Iftimie G, Pantea Stoian A, Socea B, Motofei I, Marcu D, Costache RS, Diaconu C. Complications of systemic lupus erythematosus: A review. Rom J Mil Med. 2018;121(3):9-15.
- McKay DB, Josephson MA. Pregnancy after kidney transplantation. Clin J Am Soc Nephrol. 2008:3(Suppl 2):S117-S125.
- Anantharaman P, Schmidt RJ. Sexual function in chronic kidney disease. Adv Chronic Kidney Dis. 2007;14(2):119–25.
- Palmer BF. Sexual dysfunction in uremia. J Am Soc Nephrol. 1999;10(6):1381–8.
- Saha MT, Saha HH, Niskanen LK, Salmela KT, Pasternack AI. Time course of serum prolactin and sex hormones following successful renal transplantation. Nephron. 2002;92(2):735–7.
- Lim VS, Henriquez C, Sievertsen G, Frohman LA. Ovarian function in chronic renal failure: Evidence suggesting hypothalamic anovulation. Ann Intern Med. 1980;93(1):21–7.
- Davison JM, Bailey DJ. Pregnancy following renal transplantation. J Obstet Gynaecol Res. 2003;29(4):227–23.
- Bachmann F, Budde K, Gerland M, Wiechers C, Heyne N, Nadalin S, Bachmann C. Pregnancy following kidney transplantation-impact on mother and graft function and focus on childrens’ longitudinal development. BMC Pregnancy Childbirth. 2019;19(1):1-12.
- Baylis C. Impact of pregnancy on underlying renal disease. Adv Ren Replace Ther. 2003;10(1):31-9.
- Akhtar H, Patel C, Abuelgasim E, Harky A. COVID-19 (SARS-CoV-2) infection in pregnancy: a systematic review. Gynecol Obstet Invest. 2020;85(4):295-306.
- Elhadedy MA, Marie Y, Halawa A. COVID-19 in renal transplant recipients: case series and a brief review of current evidence. Nephron. 2021;145(2):192-8.
- Waleed RM, Iftikhar W, Sehar I, Khan HS. Hematologic parameters in coronavirus infection (COVID-19) and their clinical implications. Discoveries (Craiova). 2020;8(4):e117.
- Voicu D, Munteanu O, Gherghiceanu F, Arsene L, Bohîlţea R, Grădinaru D, Cîrstoiu M. Maternal inherited thrombophilia and pregnancy outcomes. Exp Ther Med. 2020;20(3):2411-4.
- Iván Ortiz E, Herrera E, De La Torre A. Coronavirus (COVID-19) Infection in Pregnancy. Colomb Med (Cali). 2020;51(2):e4271.
- Keitel E, Bruno R, Duarte M, et al. Pregnancy outcome after renal transplantation. Transplant Proc. 2004;36(4):870–1.
- Nevers W, Pupco A, Koren G, Bozzo P. Safety of tacrolimus in pregnancy. Can Fam Physician. 2014;60(10):905-6.
- Cleary BJ, Källén B. Early pregnancy azathioprine use and pregnancy outcomes. Birth defects research part A: clinical and molecular teratology. 2009;85(7):647-54.





